Mapping Biosafety Level-3 Laboratories by Publications
I was digging as I am wont to do. It seems these topics lean from one to the next. The first part of this paper reviews the BSL-3 labs located through publications. The second part of the paper looks at the state by state BSL-4 and 3 labs in the US.
The third part of his paper reviews some of the NUTBALL research being conducted. If you think: Raccoon dog covid happened after reading this paper I’m going to look for an antenna in you to see if you are communicating with ‘alien motherships’ the pentagon thinks might land. I thought we defeated that psyop. Onto what is really happening on Mother earth with tax payer funding and against our interest.
Citation Caroline Schuerger, Sara Abdulla, and Anna Puglisi, "Mapping Biosafety Level-3 Laboratories by Publications" (Center for Security and Emerging Technology, August 2022). https://doi.org/10.51593/20220019
“Biosafety Level-3 laboratories (BSL-3) are an essential part of research infrastructure and are used to develop vaccines and therapies. The research conducted in them provides insights into host-pathogen interactions that may help prevent future pandemics. However, these facilities also potentially pose a risk to society through lab accidents or misuse. Despite their importance, there is no comprehensive list of BSL-3 facilities, or the institutions in which they are housed. By systematically assessing PubMed articles published in English from 2006-2021, this paper maps institutions that host BSL-3 labs by their locations, augmenting current knowledge of where high-containment research is conducted globally.”
This analysis is limited in that it does not include BSL-3 laboratories that do not publish their research in PubMed Central in English. They must also self-report using BSL-3 biocontainment methods in the methodology section of their publication in order to be identified by this paper.
The paper identified 148 BSL-3 institutions which includes federal research centers, universities, and companies distributed across the United States.
According to the paper, the Chinese government “directs research, including biotechnology, to meet its strategic goals and address the economic and societal needs in China. Policies such as the Medium- and Long-Term Plan for Science and Technology (S&T) Development (2006-2020) and the 13th Five Year Plan for S&T Innovation directly outline steps to develop biotechnology, including exponentially increasing R&D funding investments and establishing innovation hubs.”
China has outlined specifically, infectious diseases and public health are among the central government’s research priorities.
“The S&T plans indicate that China continues to build laboratory capacity to meet its strategic goals, and invest in major programs, which could be why they have so many labs. In contrast to the United States, our data shows the types of BSL-3 institutions in China are homogenous, reflecting a centralized system of R&D. These institutions consist mainly of state-controlled institutions, such as State Key Laboratories and People’s Liberation Army military hospitals, as well as research universities that have close ties to the central government.”
“After the United States and China, there is a steep drop-off in BSL-3 publishing institutions elsewhere. India, Germany, Spain, France, Japan, South Korea, Italy, and the Netherlands all have between 8 and 18 BSL-3 publishing institutions.
“We identified only four institutions in Russia that published BSL-3 work in English. All of these institutions are federal research centers. This may reflect a different threshold for publishing in this field, a preference for publishing in the Russian language or an emphasis on research that is either classified or for military purposes.”
This is an interesting admission that the research can be for MILITARY PURPORSES.
“Individual European countries have few BSL-3 institutions, but together they represent about a quarter of all institutions globally. Germany, Spain, France, Italy, and the Netherlands have over eight BSL-3 institutions, with the remaining 13 locations represented in the data having at least one BSL-3 institution”.
No specific mention is made of Ukraine. But then those labs might not have publishing goals in Pub Med.
“Of 157 BSL-3 institutions in North America, 148 institutions are located in the United States, while the remaining are located in Canada, Mexico, and Haiti. The BSL-3 research and diagnostic institution in Haiti, GHESKIO, is affiliated with Weill Cornell Medicine in New York.”
“Asia has 18 different locations in which we identified BSL-3 institutions, with China making up the bulk of the total count. Africa has 11 countries represented, with South Africa containing the most BSL-3 institutions in the region with a total of seven. South America has five countries represented in our analysis, with Brazil contributing five BSL-3 institutions. Australia has three separate publishing institutions, and is the only location in Oceania found in our analysis.”
“The Government Accountability Office counted over 1,300 BSL-3 laboratories registered with the Select Agents Program, and a USA Today investigation found over two hundred total BSL-3 and -4 laboratories around the United States..” I will be reviewing those counts in the next part of this substack.
The following are the total counts of BSL- 3 discovered in this paper
United States 148
China 69
India 18
Germany 17
Spain 17
France 14
Japan 12
South Korea 12
Italy 9
Netherlands 8
South Africa 7
Sweden 7
Canada 7
Belgium 6
United Kingdom 5
Brazil 5
Indonesia 4
Taiwan 4
Malaysia 3
Turkey 3
Denmark 3
Hungary 3
Switzerland 3
Australia 3
Argentina 3
Kenya 2
Uganda 2
Zambia 2
Singapore 2
Thailand 2
Austria 2
Croatia 2
Finland 2
Peru 2
* 1 The locations with one BSL-3 publishing institution were: Cameroon, Cambodia, Chile, Czech Republic, Egypt, Haiti, Kazakhstan, Mali, Mexico, Morocco, Mozambique, Nepal, Norway, Oman, Pakistan, Philippines, Poland, Portugal, Saudi Arabia, Senegal, Sierra Leone, Venezuela, Vietnam.
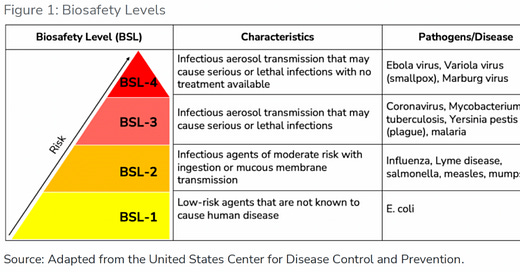
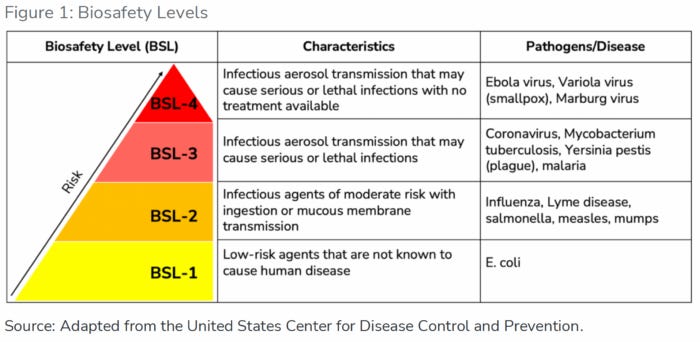
This is quite a lot of labs and countries that are working with : CORONOVIRUS, MYCOBACTERIUM, TUBERCULOSIS, YERSINIA PESTIS (PLAGUE), AND MALARIA.
THIS SITE ALLOWS YOU as a US citizen to view the biolabs ‘in your back yard’ by state in including BSL-3 and BSL-4 (Ebola, Variola virus (small pox) and Marburg virus)
A USA TODAY Network investigation identifies more than 200 biosafety level 3 and 4 lab facilities that work with dangerous pathogens - and reveals safety records that some of them fought to keep secret.
You can view each state on the site but their ‘read story’ link didn’t work for me.
Facilities that include BSL-3 or BSL-4 labs identified by USA TODAY’s research. Additional “high-containment” labs may also exist in this state.
BSL-3 Harlingen , Texas South Texas Laboratory, Texas Department of State Health Services
BSL-3 Austin , Texas Texas Department of State Health Services
BSL-3 College Station , Texas Texas A&M University
BSL-3 Dallas , Texas University of Texas Southwestern Medical Center
BSL-3 El Paso , Texas University of Texas-El Paso
BSL-3 Fort Hood , Texas Carl R. Darnall Army Medical Center Laboratories
BSL-4 Galveston , Texas Galveston National Laboratory
BSL-3 Houston , Texas Methodist Research Institute
BSL-3 Houston , Texas University of Texas Health Science Center
BSL-3 Lubbock , Texas Texas Technological University
BSL-4 San Antonio , Texas Texas Biomedical Research Institute
BSL-3 San Antonio , Texas University of Texas-San Antonio
BSL-3 Austin , Texas University of Texas-Austin
BSL-3 Amarillo , Texas Texas A&M Veterinary Medical Diagnostic Laboratory
I chose New York as well as it was also shaded dark to indicate it had a lot of labs or perhaps labs that were rated BSL-4. Think of how densely populated some of these places are.
Albany , New York Albany Med BSL-3
Albany , New York Wadsworth Center BSL-3
New York , New York Albert Einstein College of Medicine BSL-3
Buffalo , New York State University of New York at Buffalo BSL-3
Ithaca , New York Cornell University BSL-3
New York , New York Center for Infection and ImmunityBSL-3
New York , New York Icahn School of Medicine at Mount Sinai BSL-3
New York , New York New York University School of Medicine BSL-3
New York , New York Rockefeller University BSL-3
New York , New York Weill Cornell Medical College BSL-3
Plum Island , New York Plum Island Animal Disease Center BSL-3
Rochester , New York University of Rochester BSL-3
Stony Brook , New York Stony Brook BSL-3
Jamaica , New York U.S. Food and Drug Administration, BSL3
MARYLAND HAS 4 BSL-4 LABS
Facilities that include BSL-3 or BSL-4 labs identified by USA TODAY’s research. Additional “high-containment” labs may also exist in this state.
BSL-3 Baltimore , Maryland Maryland State Public Health Laboratory
BSL-3 Edgewood , Maryland U.S. Army Edgewood Chemical Biological Center
BSL-3 Baltimore , Maryland University of Maryland-Baltimore
BSL-3 Baltimore , Maryland Paragon Bioservices
BSL-3 Bethesda , Maryland National Institutes of Health
BSL-3 Silver Spring , Maryland Walter Reed Army Institute of Research
BSL-4 Fort Detrick , Maryland National Biodefense Analysis and Countermeasures Center
BSL-4 Fort Detrick , Maryland U.S. Army Medical Research Institute of Infectious Diseases
BSL-4 Fort Detrick , Maryland NIAID Integrated Research Facility
BSL-3 Frederick , Maryland Southern Research Institute
BSL-3 Rockville , Maryland BIOQUAL
BSL-3 Rockville , Maryland Sequella
BSL-3 Silver Spring , Maryland FDA Life Sciences-Biodefense Laboratory Complex
BSL-3 Rockville , Maryland MRIGlobal
BSL-3 College Park , Maryland University of Maryland, College Park
BSL-3 Laurel , Maryland U.S. Food and Drug Administration
Under the USA TODAY study you can randomly or methodically pull and review the studies, adverse events the ‘safety incidents’ or the research identified at these labs. ITS A BIT NUTS REALLY.
https://www.documentcloud.org/documents/1698038-md13.html
I pulled for instance these records. which is pages and pages of this:
POX VIRUS INSERTED LIKE PUZZLES PIECES.
“RD-12-XII-12 Submitted by Dr. Patricia Earl
Primary Reviewer: Dr. Blackstone
Secondary Reviewer: Dr. Craigie
The NIAID investigators aim to use Vaccinia virus to examine the role of A-type inclusion6 bodies (ATI) in the pathogenesis of poxviruses in the mouse model of infection. Prokaryotic work involves amplifying ATI genes from Cowpox virus and inserting them into Vaccinia virus by standard methods of homologous recombination. In eukaryotic experiments, the investigators will use HeLa and BSC-1 cells to propagate recombinant virus. Poxvirus/host interactions will be studied in the African Dormouse poxvirus model.
The Committee unanimously approved RD-12-XII-12. The Committee requests that the investigators indicate what significant aerosol-generating procedures will be performed.
Research using prokaryotic hosts is approved at BSL-1 under Section III-D-2-a of the NIH Guidelines.
Research in eukaryotic cells is approved at BSL-2 under Section III-D-3-a.
Animal work is approved at ABSL-2 under Section III-D-4-a.”
OR THIS ONE! working with LENTIVIRAL VECTORS
“Lentivirus is a genus of retroviruses that cause chronic and deadly diseases characterized by long incubation periods, in humans and other mammalian species. The genus includes the human immunodeficiency virus (HIV), which causes AIDS.” Wikepedia
“RD-12-XII-10
Submitted by Dr. Lawrence Brody Primary Reviewer: Dr. Malech Secondary Reviewer: Dr. Rao
The NHGRI investigators are using second-generation lentiviral vectors to study genetic determinants of vitamin B12 levels. They will use shRNAs to knock down various gene products in several human cell lines. The Committee unanimously approved RD-12-XII-10. Research using prokaryotic hosts is approved at BSL-1 under Section III-D-2-a of the NIH Guidelines. Research in eukaryotic cells is approved at BSL-2 with 3 practices under Section III-D-3-a.”
Or this: USING GENE ENGINEERED LYMPHOCYTES
“Adverse Event Reports RD-07-XII-17
Primary Reviewer: Dr. Malech Secondary Reviewer: Dr. Blackstone The Committee reviewed an updated SAE (autopsy report) from Dr. Steven Rosenberg dated November 30, 2012, for RD-07-XII-17, “
Phase II Study of Metastatic Cancer that Expresses NYESO-1 Using Lymphodepleting Conditioning Followed by Infusion of Anti-NY ESO-1 TCRGene Engineered Lymphocytes.”
The patient’s death on study from multi-organ failure in the setting of E. coli sepsis with neutropenia was likely related to chemotherapy and IL-2 and possibly related to cell administration[OF GENE ENGINEERED LYMPHOCYTES]. The Committee reviewed and accepted the report.
or this
Clinical Trial Reviews by the Committee RD-13-I-10 Submitted by Dr. Ravi Madan Primary Reviewer: Dr. Blackstone Secondary Reviewer: Dr. Malech The NCI investigators propose a clinical trial titled “A Phase 2 study of GI-6207 in patients with recurrent medullary thyroid cancer.” The primary objective of this randomized trial is to assess whether use of GI-6207, an immune-stimulating therapy, results in reductions of the growth rates of tumors in 34 minimally symptomatic patients with evaluable metastatic medullary thyroid cancer. Patients will be randomized to either GI-6207 for 1 year or 6 months of surveillance followed by GI-6207 for 1 year. GI-6207 will be administered subcutaneously at 4 sites at a dose of 10 yeast units per site, biweekly for 7 visits, then monthly up to 1 year of treatment. GI-6207, supplied by GlobeImmune, Inc., is a heat-killed, recombinant yeast-based vaccine engineered to express the full-length human carcinoembryonic antigen (CEA), with a modified gene coding sequence to code for a single amino acid substitution (asparagine to aspartic acid) at the native protein amino acid position 610, which is designed to enhance immunogenicity (THIS TYPE OF AMINO SUBSTITUTION HAVE WE SEEN THAT IN COVID VIRUS? LAB WORK)
Dr. Blackstone asked for a summary of the general risks associated with the yeast vaccine and any history of severe reactions. Dr. Madan stated that injection site reaction is the primary risk; thus, all patients undergo yeast skin testing prior to receiving vaccine. In the Phase 1 study they also had one patient who appeared to have an “overzealous” immune response. This patient had medullary thyroid cancer and had responded well by 3 months.
However, she went on to develop pleural and pericardial effusions and shortness of breath. Notably, the patient had 3-centimeter pericardial and pleural lesions when she was enrolled on the trial. The investigators ruled out infection and disease progression. After treating her for 36-48 hours with high-dose steroids her symptoms resolved, and in a follow up chest CT the fluid was gone. Based on that experience, this study will exclude patients with large pleural and pericardial lesions. In addition, the investigators plan to be more aggressive in using steroids should any patients exhibit a strong immune response.
Finally, Dr. Madan noted that they have not observed any autoimmune toxicity with this vaccine. The Committee unanimously approved RD-13”
OR THIS ONE: PAY ATTENTION TO THE FOWLPOX VACCINE AND THE CONTRA-INDICATIONS FOR THE STUDY !!!!! NO ONE WITH CHILDREN UNDER 3 AT HOME, PREGNANT ‘PEOPLE’ OR HISTORY OF TRANSPLANTS (HAVEN’T WE SEEN THE REJECTION OF TRANSPLANTS AFTER COVID VACCINES) OR AUTO-IMMUNE DISEASES. HAVEN’T WE SEEN THE WILDFIRE OF AUTOIMMUNE REACTIONS (IN MY FAMILY YES).
RD-13-I-11 Submitted by Dr. Ravi Madan Primary Reviewer: Dr. Blackstone Secondary Reviewer: Dr. Malech The NCI investigators propose a clinical trial titled “A Randomized Phase II Trial Combining Vaccine Therapy with PROSTVAC/TRICOM and Enzalutamide vs. Enzalutamide Alone in Men with Metastatic Castration Resistant Prostate Cancer.” The primary objective is to determine if PSA-TRICOM combined with enzalutamide will increase time to progression (TTP) in patients with chemotherapy-naïve, metastatic, castration-resistant prostate cancer (mCRPC) compared to enzalutamide alone. The study will randomize chemotherapy-naïve mCRPC patients to either enzalutamide alone or enzalutamide with PSA-TRICOM. Enzalutamide will be given at the standard dose of 160 mg daily. PSA-TRICOM will be administered at week 1 (vaccinia-PSATRICOM, 2x108 units subcutaneously) and then week 3, 5, and then monthly fowlpox vaccine (2x109 units subcutaneously). After completing 6 months of vaccine, fowlpox vaccine (2x109 units subcutaneously) will be administered every 3 months based on previous clinical trial experience with PSA-TRICOM. The primary endpoint of this trial will be TTP, with secondary endpoints including overall survival, and exploratory analysis evaluating immunologic responses to vaccine with enzalutamide and to enzalutamide alone. Fowlpox-PSA(L155)/TRICOM is a recombinant fowlpox virus vector vaccine containing the genes for human PSA and three co-stimulatory molecules: B7.1, intercellular adhesion molecule- 5 1 (ICAM-1), and leukocyte function-associated antigen-3 (LFA-3). VacciniaPSA(L155)/TRICOM is a recombinant vaccinia virus vector vaccine containing the genes for human PSA and three co-stimulatory molecules: B7.1, ICAM-1, and LFA-3. Dr. Madan explained that safety concerns for the study primarily relate to handling procedures for the vaccinia-based vaccine. However, their pharmacy team is experienced in handling the vaccine and educating patients. In addition, the trial excludes patients who have children under age 3 at home, pregnant people at home, or a personal history of transplants or autoimmune disease. The Committee unanimously approved RD-13-I-11.
OR THIS. EVER HEAR PEOPLE NOT WANT TO TAKE SWABS UP THEIR NOSES? WELL READ ON.
“Reviews by the Committee RD-13-I-13 Submitted by Dr. Deborah Citrin Primary Reviewer: Dr. Hoyt Secondary Reviewer: Dr. Craigie The NCI investigators aim to determine whether the IGF gene plays an important role in radiation injury.
They will treat mice with intranasal exposure to adenovirus serotype 5 encoding GFP +/- Cre recombinase. In mice exposed to the virus with Cre recombinase, Cre expression will allow knockout of floxed genes in the host.
They will use this model to test the importance of several pathways in radiation lung injury by exposing mice to radiation after treatment with Adeno-Cre. No prokaryotic or eukaryotic work is proposed. The investigators will obtain the pacAd5 CMV eGFP vector and the pAd5CMVCre-eGFP from the University of Iowa Gene Transfer Vector Core and
deliver it intranasally to mice without further modification. The Committee unanimously approved RD-13-I-13.”
HOW ABOUT THIS FRANKINSTEIN STUDY INJECTING VIRUS INTO BRAINS?
“RD-13-I-18 Submitted by Dr. Donna Calu Primary Reviewer: Dr. Hoyt Secondary Reviewer: Dr. Craigie The NIDA investigators are studying the role of dorsal medial prefrontal cortex (mPFC) and its afferent regions in yohimbine-induced reinstatement of food seeking. The investigators propose to use a novel method, involving use of viruses to induce expression of receptors, to inactivate the important circuits identified in previous experiments.
These receptors, known as Designer Receptors Exclusively Activated by Designer Drugs (DREADD), have no function in the normal brain, and they can only be activated by an experimentally delivered drug, Clozapine NOxide (CNO).
The investigators will first perform intracranial injections of AAV-hSyn-hM4D(Gi)- mCitrine (the control groups will be injected with the control construct AAV-hSyn-EGFP) to inhibit neurons in the dorsal mPFC and other potential regions of interest in the brains of rats. This would allow neurons in the targeted areas to selectively express DREADDs.
Subsequently, intraperitoneal injections of CNO will selectively inhibit neurons in these regions. The investigators will then examine the impact of inhibiting neuronal populations on stress-induced reinstatement of food seeking using a rat reinstatement procedure. No prokaryotic or eukaryotic work is proposed: Constructs will be produced by University of North Carolina vector core or NIDA Optogenetic and Transgenic Technology core. The Committee unanimously approved RD-13-I-18. ”
WE FOUND THE BAT, DOG MATING RACCOON. IF I HEAR IT HAPPNED IN NATURE ONE MORE TIME AFTER READING THIS STUFF!!!! These labs use animals or people; they test viruses and vaccines. Nature? No the Lab Leak theory must be true.
“Amendments Amendment to RD-04-X-03 Submitted by Dr. Jack Bennink Primary Reviewer: Dr. Craigie Secondary Reviewer: Dr. Rao The NIAID investigators request approval of an amendment to add an ASP in guinea pigs to study influenza virus transmission. The Committee conditionally approved this amendment, pending clarification of the following issues: Reference to injecting animals with “whole virus” on page 2 of the ASP; Whether animals are going to be challenged. In the event that a challenge with influenza virus is planned, the ABSL will be determined by the challenge virus. Animal work is approved at ABSL-2 under Section III-D-4-a of the NIH Guidelines.
I stopped looking at page 22 of 297 of the FIRST DOCUMENT I RANDMONLY PULLED UP. If you want to know the type of research taking place, the adverse events they are recording and the specific interest work taking place you can explore through the USA Today link provided above. This might be a great spot for the Children’s Defense to get into WITH THEIR TEAMS.
If Congress thinks Wuhan is the hot bed, they really need to get into this reporting ON THEIR OWN SOIL while its still up. It’s absolutely endless.
Wow remember when reporters worked HARD in the interest of the people. Oh wait, that’s us on substack. Here I am relentless. On a hunt.
Please give us your support. If you like my work lean in, and share, subscribe or upgrade your subscription. I appreciate your readership. I cover financial, science, legal, political, philosophical. I’m sometimes emotional, sometimes angry. Always flawed, hopeful, trying and learning.


I didn't see the US labs in Ukraine on the list. That's where they make jabs targeted to a certain ethnicity. Here's a map of Ukraine and another of the greater Africa/Europe/Asia region showing all US biolabs in those areas.
https://secularheretic.substack.com/p/pharmacogenomics-toxicogenomics
NB: The first line should read: "...as I am wont to do."