Abstract
“Medical microrobotics is an emerging field that aims at non-invasive diagnosis and therapy inside the human body through miniaturized sensors and actuators. Such microrobots can be tethered (e.g., smart microcatheters, microendoscopes) or untethered (e.g., cell-based drug delivery systems). Active motion and multiple functionalities, distinguishing microrobots from mere passive carriers and conventional nanomedicines, can be achieved through external control with physical fields such as magnetism or ultrasound. Here we give an overview of the key challenges in the field of assisted reproduction and how these new technologies could, in the future, enable assisted fertilization in vivo and enhance embryo implantation. As a case study, we describe a potential intervention in the case of recurrent embryo implantation failure, which involves the non-invasive delivery of an early embryo back to the fertilization site using magnetically-controlled microrobots. As the embryo will be in contact with the secretory oviduct fluid, it can develop under natural conditions and in synchrony with the endometrium preparation. We discuss the potential microrobot designs, including a proper selection of materials and processes, envisioning their translation from bench to animal studies and human medicine. Finally, we highlight regulatory and ethical considerations for bringing this technology to the clinic.
“n vivo imaging of microrobots is challenging in general because of their small size and the scattering properties of the tissue. Suitable imaging modalities can be classified by the mechanisms of contrast that they use: either optical, magnetic, mechanical, or due to radioactive decay. The imaging approach defines the spatial resolution, the penetration depth as well as compatibility with clinical practice, which can be considered the most relevant properties for in vivo applications. A comprehensive overview of different techniques is given by ref. 50. For example, infrared (IR) imaging is appealing for ophthalmology and sub-skin interventions as the penetration depth of light in tissue is comparably small. For applications like small animal imaging, with cm penetration depth, other techniques should be employed, such as US and photoacoustics. We showed for the first time real-time tracking of single moving micro-objects below cm thick phantom tissue and ex vivo chicken breast, using PAI51. The resulting PA signal was further improved in terms of contrast and specificity by coating the micro-object surface with gold nanorods. This coating possesses a unique absorption spectrum, which facilitates its discrimination from surrounding biological tissues when translated to future in vivo settings.
For operating at a human scale, imaging techniques like magnetic resonance imaging (MRI), nuclear techniques like positron emission tomography (PET), or single-photon emission computed tomography (SPECT) are established as diagnostic tools. However, their use in surgical procedures is hampered by the cost and clinical practicability as well as the exposure to ionizing radiation in the cases of PET and SPECT.
We predict that in the context of clinical applications, US-based modalities with contrast enhancement will play a central role in the real-time imaging of microrobots. US imaging, in general, can achieve high penetration depths in tissues while avoiding exposure to ionizing radiation. This, as well as its widespread clinical acceptance, cost efficiency, and flexibility, make it a great tool for microsurgical interventions. However, due to typical wavelengths in the millimeter range, it cannot sufficiently resolve microrobots. Therefore, US should be combined with different contrast-enhancing agents, exploiting the non-linear acoustical properties of microbubbles for contrast-enhanced US (CEUS)52, optical adsorption for multispectral optoacoustic tomography (MSOT) and PAI, a different movement reacting to a magnetic field for magneto-motive US (MMUS), or active beacons with coded responses53.
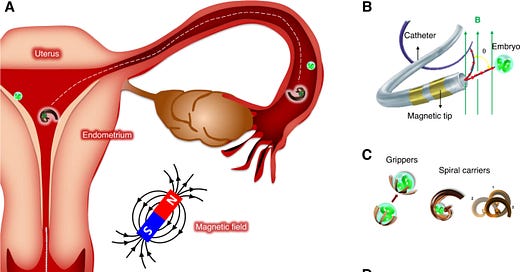
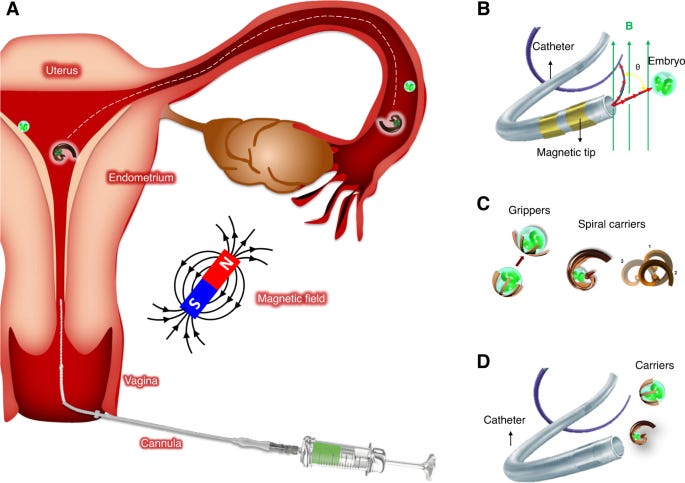
Remote-controlled microsystems need to be externally provided with propulsion and guidance toward a target with sufficient force/momentum. A common approach is to have magnetic microrobots that react to the field of external permanent magnets or electromagnets. A torque exerted from the magnetic field can change the orientation of magnetic microrobots and catheters for steering. Furthermore, a forward motion can be generated by rotating helical-shaped objects. Another mechanism for propulsion is gradient pulling, where a magnetic field gradient can exert a direct force on magnetic microrobots. Typically, the applied magnetic field strength is in the order of a few mT, which is almost three orders of magnitude less than that in clinical magnetic resonance imaging (MRI) devices. Exposure to magnetic fields of this strength is considered safe in general, even prenatal exposures to a magnetic field of 1.5 T during the second and third trimester of pregnancy in a cohort of 72 healthy fetuses showed no adverse effects on birth weight, long-term neurodevelopmental outcomes, growth, motor functioning, social or neurological development54,55.”
What is also interesting in this article is the ‘ethical considerations and barriers reviewed’.
Ethical and regulatory considerations for a clinical application
“The development of microrobots toward clinical applications in reproductive medicine requires addressing several distinct ethical and regulatory aspects:
i.
The potential use of novel technology/medical devices in reproductive medicine and gynecology has to be balanced against potential risks/advantages and existing alternative approaches. More delicately, the potential targeting of biomaterial containing cellular constructs towards germ cells may interfere with the strict embryonic protection laws in place in several countries (e.g., Germany, Federal Law Gazette, Part I, No. 69, issued in Bonn, 19 December 1990, page 2746). In such cases, ex vivo applications may be advisable.
ii.
Ethical commissions might be more willing to discuss the clinical use of such novel and complex therapeutic approaches in life-threatening diseases like cancer or other injuries in the reproductive system. In such indications, an ethical board approval may be willing to consider e.g., the targeted delivery of compounds towards malignant cells/undesired tissue growing.
iii.
Again, most authorities would classify the non-biological part of a microrobot as a “medical device” and would follow the respective regulatory pathway (e.g., EU directive for medical devices)62. The lowest risk category I, accordingly would apply to devices that can be used with no or very low risk for a human being (e.g., diagnostic testing). All applications in which microrobots would be injected into body openings or fluid would be categorized II or III with consecutively higher hurdles for licensure.
iv.
The combination of a medical device with a pharmaceutical compound or a living cell would be seen as a ‘combined’ product from the regulatory perspective. This results in a more complex approval procedure as existing knowledge regarding the bio-distribution, preclinical safety and toxicity for each separate component can not be used directly. Thus, a full risk-benefit assessments has to be conducted for novel combined products.
v.
It can be assumed that using this technology (considered minimally invasive due to the small size of medical microrobots), the use of general anesthesia is not required.
vi.
The possible administration route of microrobots into the human body in the case of embryo/gamete transfer could be in analogy to the vaginally performed and possibly ultrasound-guided embryo transfer or artificial insemination.
vii.
The microrobots must fulfill existing standards including sterility comparable to IVF culture media used for embryo transfer. In analogy to IVF, culture media studies have to be performed before introducing them to humans.
viii.
International regulatory authorities like FDA or EMA have started developing position statements and regulatory frameworks trying to address the increasing interest in the application of therapeutics containing nanomaterials (FDA Nanotechnology Task Force, “Nanotechnology Task Force Report 2007,” at ii (July 25, 2007))63, which should be also taken into consideration of the proposed microrobots/carriers decorated with nanomaterials for example for enhanced imaging contrast or combined therapies.
Keeping the aforementioned challenges in mind, it seems highly advisable to involve national and international competent regulatory authorities (e.g., Paul-Ehrlich Institute, EMA, FDA) as early as possible throughout the translational development of a micro/nanobot application.
Several national authorities offer “scientific advice” to researchers, to determine prerequisites for preclinical safety testing (e.g., large animal data, stability, tumorigenicity, etc.). More recently, this advice can even be obtained from several national authorities in parallel64.
Together with clinical research organizations (CRO), this will allow academic organizations or start-up companies to save costs when developing and applying for “first-in-human” microrobot applications.”
One has to consider just how fast science is moving and how slow the ethical considerations and disclosure to the population is.