The referral of the mRNA technology as Nanomedicines may be the most apt description absent ‘bioweapons’. But certainly more apt then ‘vaccine’. This paper is important for laying out plainly the foundations of mRNA as nanotechnology and nanomedicines.
It refers to the contents of the LPN or the nanomedicines as ‘payloads’. was this a slip? Payloads are used usually in nanomedicines to talk about highly toxic cargoes, FOR instance as ADC payloads
“Antibody-drug conjugates (ADCs) are an emerging class of pharmaceuticals designed to deliver highly toxic cargoes (payloads) to clinical targets. In ADCs, payloads are the pharmaceutically active component. For this reason, they dictate the overall effectiveness of these therapies. Up to recently, the development of payloads for ADCs has focused almost exclusively on cytotoxic drugs and cancer targets. But as the safety and efficacy of linker chemistry continue to increase, so does the diversity of payloads that can be used for the generation of new ADCs”
The paper indicates nanotechnology is behind the ‘success of mRNA vaccines for Covid-19’. THE nomenclature even shifts to call them ‘nano-vaccines’ and nanomedicines. So unequivocally the published we can set aside the issue of ‘conspiracy’ that there is nanotech in the mRNA vaccines. The words of ‘success’ or any other of the ‘narrative’ and ‘ideology’ exclamations are those of the authors of the paper reproduced below and not my own. Our significant departure on either the ‘success’ at preventing transmission or the narrative of safe is so significant in peer reviewed literature, although strangely (and other things that go hum) it is still not reflected in our discourse publicly and through MSM and big tech. This is one of the largest indications of collusion between government, media, big tech and pharma. So it is an interesting visible view that these act as one. I previously referred to them as the Amoeba. This would be an interesting area of peer reviewed publication: all the times the media big tech ignored very important medical literature criticizing the nanomedicines.
The use of payloads to refer to the mRNA is in the last sentence.
Shall we just jump in. I’ve been writing so much lately that my coffee gets cold before an essay is finished.
Role of nanotechnology behind the success of mRNA vaccines for COVID-19
Amit Khurana,a,b,c,d,⁎,1 Prince Allawadhi,e,1 Isha Khurana,f Sachin Allwadhi,g Ralf Weiskirchen,c,⁎⁎ Anil Kumar Banothu,b,i Deepak Chhabra,h Kamaldeep Joshi,g and Kala Kumar Bharanid,i,⁎⁎⁎
Author information Article notes Copyright and License information Disclaimer
Graphical Abstract
Keywords: COVID-19, mRNA, Lipid nanoparticles, BNT162b2, mRNA-1273
Abstract
The emergency use authorization (EUA) by the US-FDA for two mRNA-based vaccines BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) has brought hope of addressing the COVID-19 pandemic which has killed more than two million people globally.
Nanotechnology has played a significant role in the success of these vaccines. Nanoparticles (NPs) aid in improving stability by protecting the encapsulated mRNA from ribonucleases and facilitate delivery of intact mRNA to the target site. The overwhelming success of these two mRNA based vaccines with ~95% efficacy in phase III clinical trials can be attributed to their unique nanocarrier, the "lipid nanoparticles" (LNPs). LNPs are unique compared with bilayered liposomes and provide improved stability of the cargo, possess rigid morphology, and aid in better cellular penetration. This EUA is a major milestone and showcases the immense potential of nanotechnology for vaccine delivery and for fighting against future pandemics. Currently, these two vaccines are aiding in the alleviation of the COVID-19 health crisis and demonstrate the potential utility of nanomedicine for tackling health problems at the global level.
Introduction
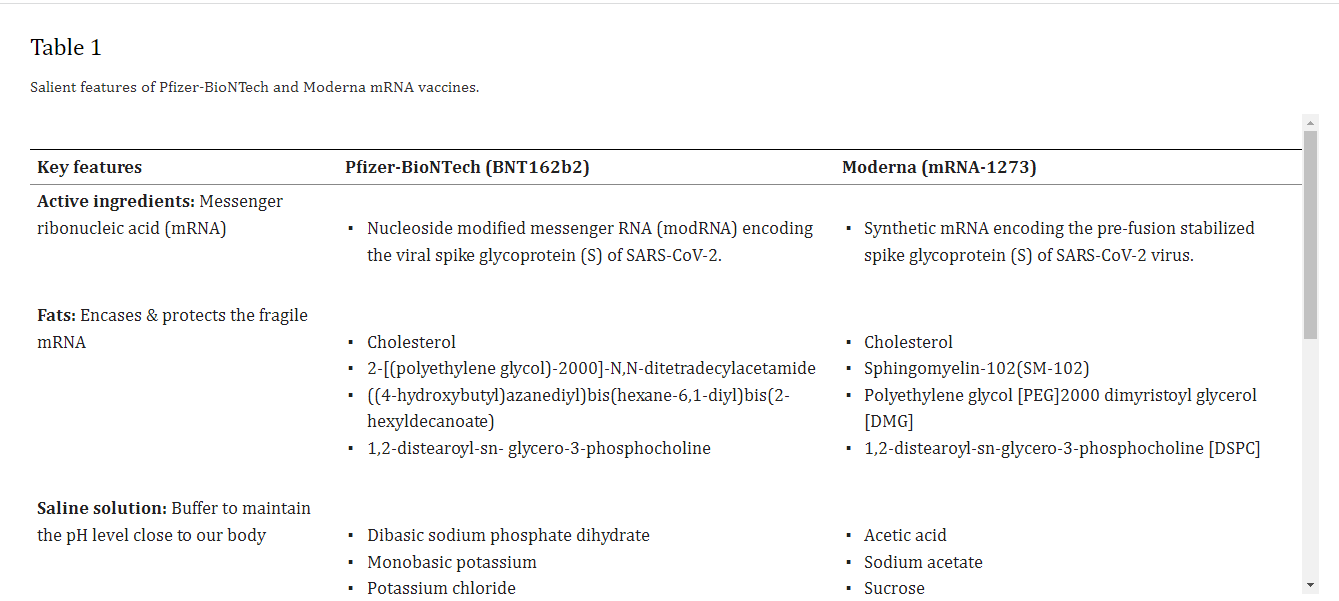
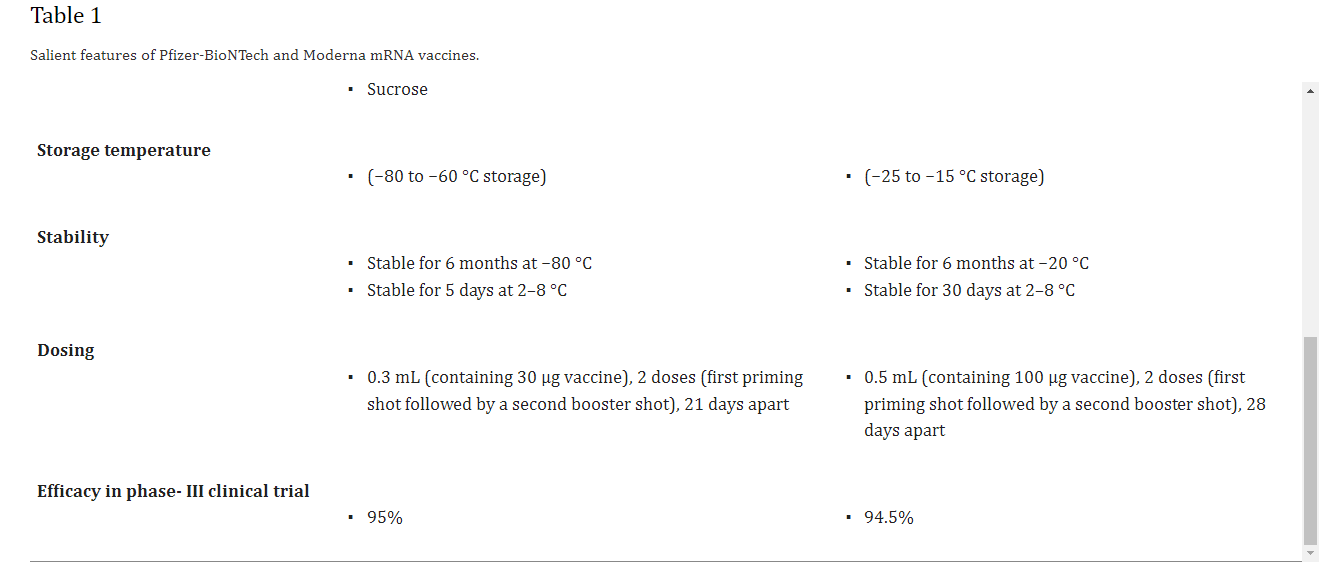
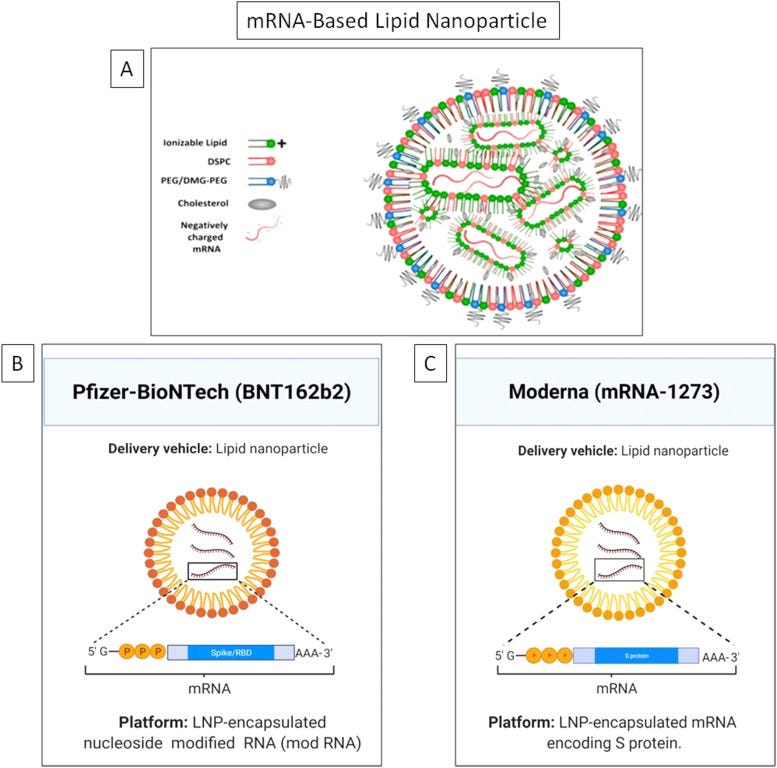
It is the first time in history that two mRNA-based vaccines developed using lipid nanoparticles (LNPs) have been given emergency use authorization (EUA) by the US FDA for clinical therapeutics against the COVID-19 [1]. This undermines the skepticism on the potential of nanotechnology based approaches. Nanoparticles (NPs) offer many unique advantages compared to other conventional drug carriers including tailored drug release profile, enhanced surface area, protection of the cargo from degradation and modulation of drug pharmacokinetics [2], [3], [4], [5], [6], [7], [8], [9]. Nanotechnology has fast tracked the development of mRNA-based COVID-19 vaccines invented by Moderna and Pfizer/BioNTech [10], [11], [12]. On 16th November 2020, Moderna officially shared the preliminary data of the phase III clinical trial of its COVID-19 candidate vaccine mRNA-1273 followed by Pfizer-BioNTech on 18th November, 2020 with the clinical trial outcome of its COVID-19 candidate vaccine BNT162b2. Efficacy is an important parameter for vaccines and is defined as the percentage reduction in disease incidence among the vaccinated group during the clinical trial compared with an unvaccinated control group under similar conditions [13]. The primary data revealed BNT162b2 and mRNA-1273 have an efficacy of 95% and 94.5% against SARS-CoV-2, respectively [14], [15]. The Moderna vaccine is based on a stabilized mRNA of the viral spike protein, [16], [17] and the BNT162b2 is based on a nucleoside modified RNA (modRNA) of the SARS-CoV-2 virus. Table 1 lists the chemical composition of both the vaccines and Fig. 1 depicts the key delivery carrier.
Table 1
Salient features of Pfizer-BioNTech and Moderna mRNA vaccines.
[efficacy was later ‘redacted’ in subsequent studies and even admissions from Fauci et al.]
Open table in a separate window
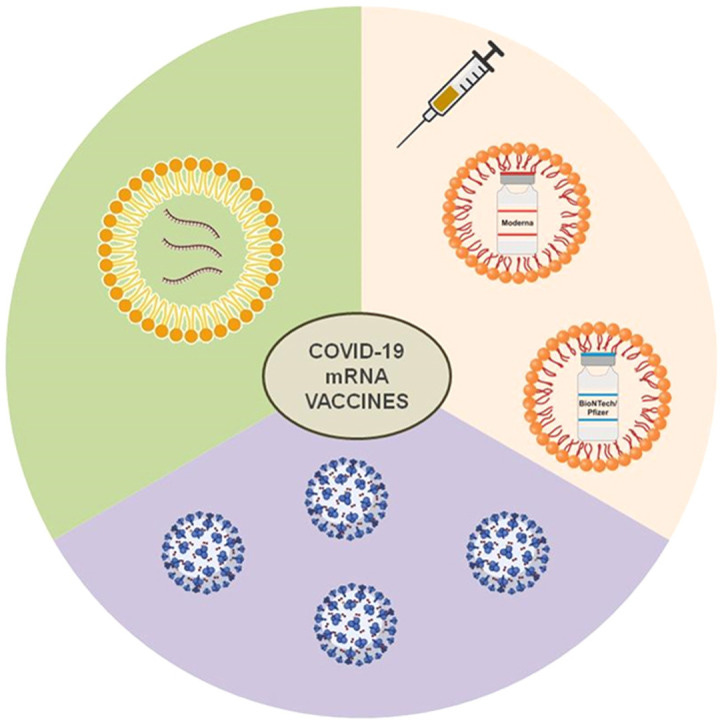
A) The general structure of lipid nanoparticle (LNP) showing the major components and the unique mRNA cargo of, B) Pfizer-BioNTech, and C) Moderna vaccines.
Figure A) Reprinted (adapted) with permission from [53] and is Copyright (2021) of American Chemical Society. Figure B) and C) were created with BioRender.com.
Over the past one year, some more vaccines have been developed and are now being used clinically on regular a basis. The major new vaccines include inactivated/live attenuated virus, recombinant protein, recombinant viral vector, DNA vaccine, and messenger RNA (mRNA)-based vaccines [7], [18].
COVID-19 vaccines based on conventional technologies include vaccines developed by Oxford/AstraZeneca (UK), Johnson and Johnson (USA), Gamaleya Research Institute (Russia), Bharat Biotech (India), SinoVac (China), Sinopharm (China) and others [19], [20], [21], [22], [23], [24], [25].
These conventional vaccines based on inactivated/live viruses offer advantages like robust immune response, ease of storage and shipping [26], while the disadvantages include difficulty in manufacturing at sufficient titers, cost per dose and the need for multiple doses to achieve immunity [27].
However, mRNA-based vaccines were granted prioritized clinical approval as the technology ensures the stability of the mRNA, together with enhanced delivery efficiency to ferry the mRNA inside the host cell. mRNA vaccines are both non-infectious, and thus safer [HAHAHAHAHA], and do not require penetration into the nucleus, which is difficult to achieve [28].
Further, these vaccines can be produced rapidly, which is a major advantage in the existing pandemic, where billions of doses are needed in a short time to vaccinate the world population [29], [30], [31], [32].
mRNA uptake by a cell is a very challenging task, firstly, due to presence of RNA degrading enzymes, which debase every RNA molecule they encounter [33], [34], and secondly, being negatively charged, mRNA cannot easily cross the negatively charged cell membrane. Hence, to address this challenge, researchers have designed LNP based carrier molecules to preserve mRNA integrity and foster its uptake inside the cell [35].
LNPs are complex systems, which aid effective delivery of siRNA or mRNA into the host cells. Being a US-FDA approved carrier, LNPs are now extensively used for delivering antigen-encoding mRNA, encapsulating viral antigens against influenza, rabies, human immunodeficiency virus (HIV), cytomegalovirus (CMV) and others [36], [37].
The focus on delivery: key advantages of lipid nanoparticles
In the late 1960s, liposomes were anticipated as a novel drug delivery system (NDDS) and since then have been improved for disease targeting [38]. LNPs present a novel colloidal drug delivery system, and differ from liposomes in that they form micellar structures within the core that can be modified based on formulation and synthesis parameters [39]. The structure of LNPs consists of a solid core made up of lipid, which is composed of triglycerides or any other glyceride mixture. A typical LNP has four parts: (i) an ionizable lipid portion that allows self-assembly, enhances the rate of mRNA encapsulation, and aids endosomal escape, (ii) a stabilizing agent for stability and membrane fusion (cholesterol or a sphingolipid), (iii) a phospholipid that stabilizes the bilayer, encapsulating the lipid structure [40], and (iv) polyethylene glycol (PEG), a lipid-based stabilizing agent that reduces nonspecific binding to proteins, increases half-life, and boosts circulation time by aiding escape from first pass metabolism or reticulo-endothelial system (RES).
Their rigid morphology and kinetic stability are key advantages, making LNPs, a carrier of choice over liposomes. Their potential to transport a diverse group of therapeutic cargos from therapeutic drugs to nucleic acids (mRNA, siRNA, DNA) making them an appealing drug delivery system [41], [42], [43]. Once mRNA enters inside the cytoplasm from the endosome, it translates into the encoded immunogenic protein against specific antigens [44], [45]. This recent discovery and integration of nanotechnology have shown various advantages in safer [HAHAHA] delivery of next generation RNA vaccines.
In addition to their simple synthesis method, small size and serum stability, the efficacy of LNPs in the delivery of nucleic acids into cells makes them superior to other carriers. Since biological membranes and nucleic acids are negatively charged, it is difficult to deliver mRNA across this barrier. LNPs offer an ideal platform for delivering nucleic acid therapies as the ionizable lipids are near-neutrally charged at physiological pH.
However, in acidic endosomal compartments (pH-4.5), they become ionized, promoting endosomal escape for effective intracellular delivery [46], [47].
[ph of the host and temperature of the product would alter how the biomedicines react with the host. ARE WE POSITING THE CHARGES IS WHAT IS PROVIDING THE attraction and COAGULATION OF PARTICLES and changing behaviour of red blood cells AND THUS THE CLOTS? WE HAVE ALSO JUST REVIEWED THE ABILITY OF THE BIOACCUMULATION OF HEAVY METALS AND OTHER PARTICULATES OF THE NANOTECH IN SHOTS']
Hence, LNPs achieve high encapsulation rate for nucleic acids with improved transfection efficiency. Furthermore, LNPs have relatively low cytotoxicity and immunogenicity compared to liposomes, thus favoring delivery of nucleic acid based therapeutics [48], [49], [50].
Lipids used in formulating these nanoparticles are biocompatible and are well endured by fatty acids, triglycerides, waxes and steroids. In addition, selecting a good combination of emulsifiers could make the formulation more stable with higher efficiency [52].
In terms of industrial scale up, LNPs offer multiple advantages compared with other carrier systems, such as large scale production using microfluidics or T-junction mixing, stability, and low cost of raw materials [52], [53], [54].
The most significant parameters in LNPs characterization are particle size, their size distribution, degree of polymorphism, zeta potential, crystallinity, drug loading, drug release and entrapment efficiency [55].
Drug release from LNPs mostly relies on the type of matrix used and the position of drug in the matrix formulation. The ingredients of lipid matrix, manufacturing parameters and surfactant concentrations, such as rate of stirring, temperature etc. can modulate the drug release profile [56]. In a nutshell, the most fundamental rationale of using LNPs, as an alternative to polymeric nanoparticles, is the simplicity of large-scale manufacturing and their low toxicity [57], [58].
The path forward
To harness the full potential of mRNA therapeutics in future, the following challenges need particular attention: (i) the intrinsic immunogenicity of mRNA; (ii) the propensity to enzymatic and thermal degradation; and (iii) the inability to cross negatively charged cell membranes. Nucleoside modification, sequence engineering by codon optimization and uridine depletion are some of the methods used to reduce inherent immunogenicity, protect from enzymatic degradation and facilitate cellular uptake of the mRNA platform [59], [60], [61], [62], [63]. The mean half-life of mRNA vaccines decreases with increasing temperature, which is a challenge for their long term storage.
However, chemical modification by applying an outer coating of nonionic or an ionic surfactant enhances the thermal stability of mRNA. These chemical alterations change the dimensions of a nanoparticle and aid in the effective carrying of mRNA with higher thermal stability.
Some of the reported allergic reactions to LNP nanovaccines can be minimized by using PEG complexed with lipids, which have improved biocompatibility.
Hence, in future mRNA-based nanovaccines should possess an improved safety profile, higher stability (for storage at higher temperature), and enhanced cellular penetration. The next generation RNA vaccines will be more personalized and the tools of genetic engineering will play a crucial role in exploiting the full potential of the mRNA platform.
Concluding remarks
The EUA of the Pfizer-BioNTech and Moderna vaccines has undoubtedly brought great hope [HAHAHAHA] for the years ahead. However, it remains challenging to decide how to prioritize the allocation of vaccines to the population. While the EUA of these mRNA vaccines brings hope for developed countries, these vaccines remain largely out of reach of developing and underdeveloped nations on economic grounds and need for special storage conditions.
At high or at room temperature, mRNA has poor stability, and thus these mRNA-based vaccines need to be stored at such a low temperature. The Pfizer-BioNTech vaccine needs to be stored at −80 °C to −60 °C and the Moderna vaccine at −25 °C to −15 °C to avoid degradation of mRNA encased inside the LNPs.
[TEMPERATURE STORAGE MAY CHANGE THE CHARGE AND PROPERTIES AND EVEN DANGER OF THE VACCINES, studies of the biomedicines properties at each possible temperature from -80 to room temperature ought to have been plotted]
Further, as it is common to all vaccines, mRNA vaccines are not devoid of side effects, including pain at the site of administration, fever, chills, fatigue, headache, muscle pain, and joint pain.
In addition, some patients have experienced allergic responses, which has been linked to the presence of PEG in the formulation [64].
The adverse effects, duration of protection and storage at very low temperature are critical issues that require careful consideration for the upcoming COVID-19 nanovaccines [54].
Nonetheless, the two mRNA vaccines represent a great success for biotechnology and molecular therapeutics. It motivates materials scientists who have developed and optimized nanoformulations for drug and vaccine delivery over the past two decades and encourages their acceptance in nanomedicines.
Until the COVID-19 crisis, oncology had been the major area where nanotechnology based drug carriers had been widely explored.
These two mRNA-based vaccine formulations will serve as a stepping stone for future applications of nanomedicine.
These nanocarrier based vaccines highlight the importance of the nanoscale and the ability of nanoscale delivery systems to protect payloads from degradation, provide tailored biodistribution and cellular delivery.
Payload has a military connotation an explosive warhead carried by a missile. Words are always important and the concept of PAYLOAD to describe the mRNA nanomedicines is apt. There are other words as effective and while it is a terminology used in some papers, they leave it at the end. They are leaving clues for us I think. hey look at the charge. hey look at the temperature. hey look at PEG. Hey this is nanotech. and last it’s a missile going in your body.


"NANOTECH AND mRNA: PAYLOADS!"
.
Well done - LawyerLisa - the truth will set us free!
.
THIS ability to transport a payload - is the KEY to their reckless "mishandling" of the situation - the gangsters around Antony Fauci just keep LYING, LYING & LYING again . . .
.
Once "We the People" accept the "benefits of mRNA", we are finished - as in ANY moment in time - they can CHANGE that payload from any "vaccine" to any STEALTH deadly kill-shot, without telling "We the People" . . .
.
THAT is why CRIMINAL PSYCHOPATH & raving WEF-SATANIST JUSTIN TRUDEAU with his KILLER-COMPANION CRYSTIA FREELAND - instead of clearing up "WHAT WENT WRONG" - with the crazy misunderstanding & mishandling of that crazy BIO-WEAPON-ATTACK on "We the People" of CANADA, with Covid-19 / SARS-Cov-2, NOW WANTS TO INSTALL MORE VACCINE MANDATES FOR THE FUTURE!
https://lionessofjudah.substack.com/p/smoking-gun-caught-on-tape-trudeau?
Well done, and scary how they openly talk about it more or less bragging. However most people or should I say sheeple won't ever hear about, and if they should be presented with the info will likely ignore it, as long as the MSM tells them it's all SAFE AND EFFECTIVE.