Should regulatory frameworks work this way? Expose is not Endorsement.
“Sep 27, 2022
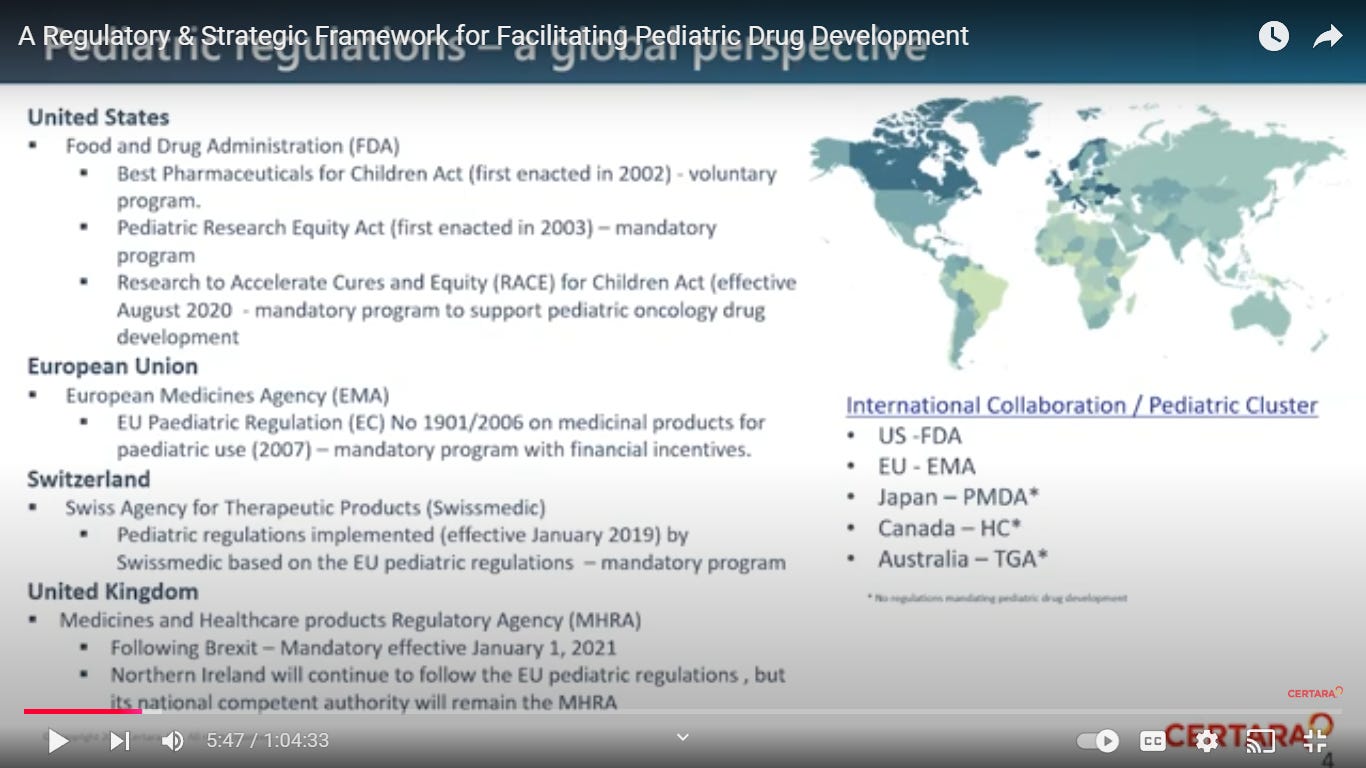
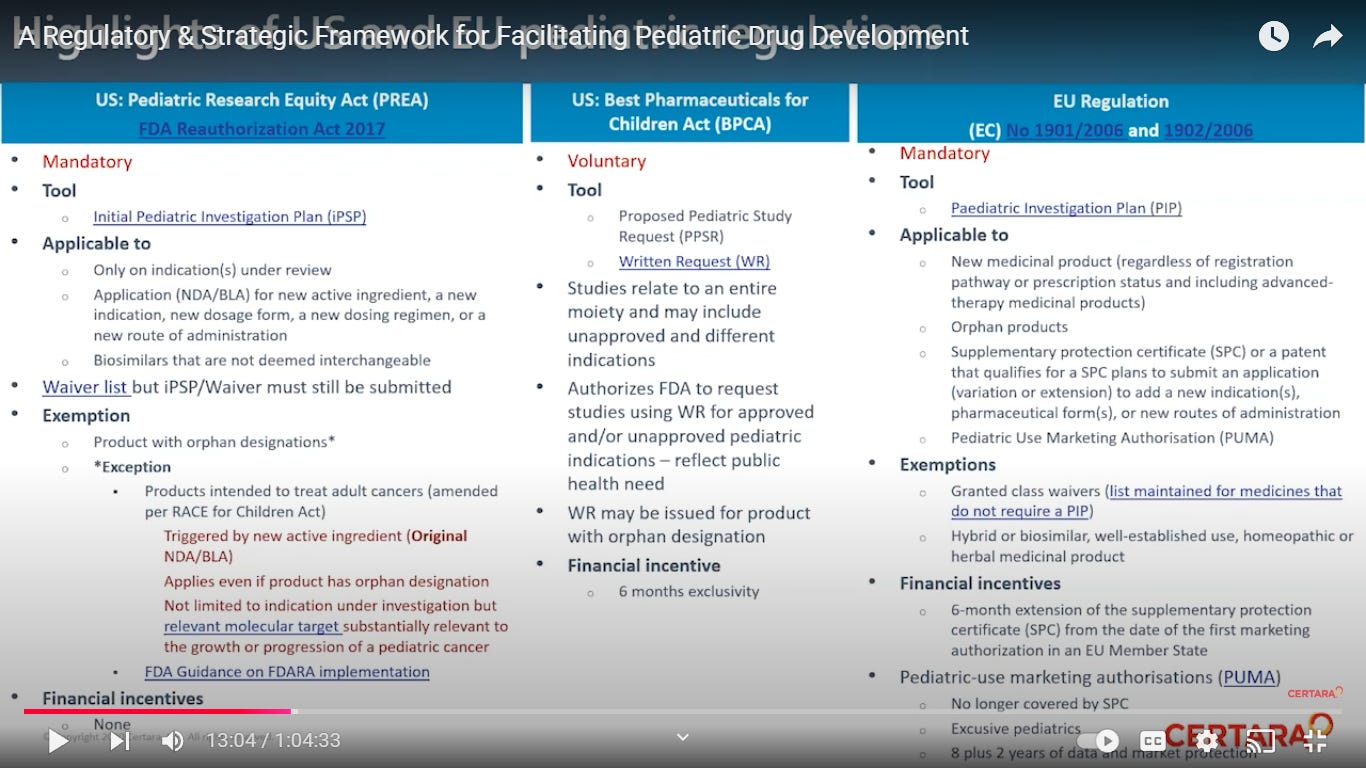
Regulations in the US and Europe require and/or incentivize sponsors to evaluate their drugs (small molecules and biologics) for use in appropriate pediatric populations.
It is generally agreed that that these regulations have stimulated new research efforts in pediatrics.
It is essential that individuals, involved in global pharmaceutical research and development, are familiar with these regulations in order to create development efficiencies and minimize product development delays and cost.
During the webinar, pediatric development experts provide an overview of the global regulatory framework and requirements that drive pediatric development.
They discuss intrinsic challenges with generating data across pediatric populations and provide case studies on how model-informed drug development (MIDD), can achieve a more efficient and more predictive clinical research process.
Finally, our experts share with you how to effectively communicate the plan to regulatory authorities.
Certara accelerates medicines to patients using proprietary biosimulation software and technology to transform traditional drug discovery and development. I
ts clients include 1,600 global biopharmaceutical companies, leading academic institutions, and key regulatory agencies across 60 countries. Please visit us at
https://www.certara.com/”
use of modelling and simulation;
regulatory agencies and stakeholders meet together to ‘harmonise regulations”
5:46 Covid 19
HERE IS A SLIDE TO READ:
I will just go ahead and expand it.
OH HEALTH CANADA MAY REQUEST, BUT CANNOT REQUIRE, A SPONSOR OR APPLICANT TO GENERATE OR SUBMIT DATA ON A DRUG’S SAFETY AND EFFICTIVENESS IN CHILDREN.
oh and pharma focal point.
extrapolate safety. hmmm.
use of orphaned products… hmm..
I recommend everyone interested in ‘regulatory’ review of pediatric drug development.
MODELLING AND SIMULATION; is not proving anything is safe.
Incentive programs. good?
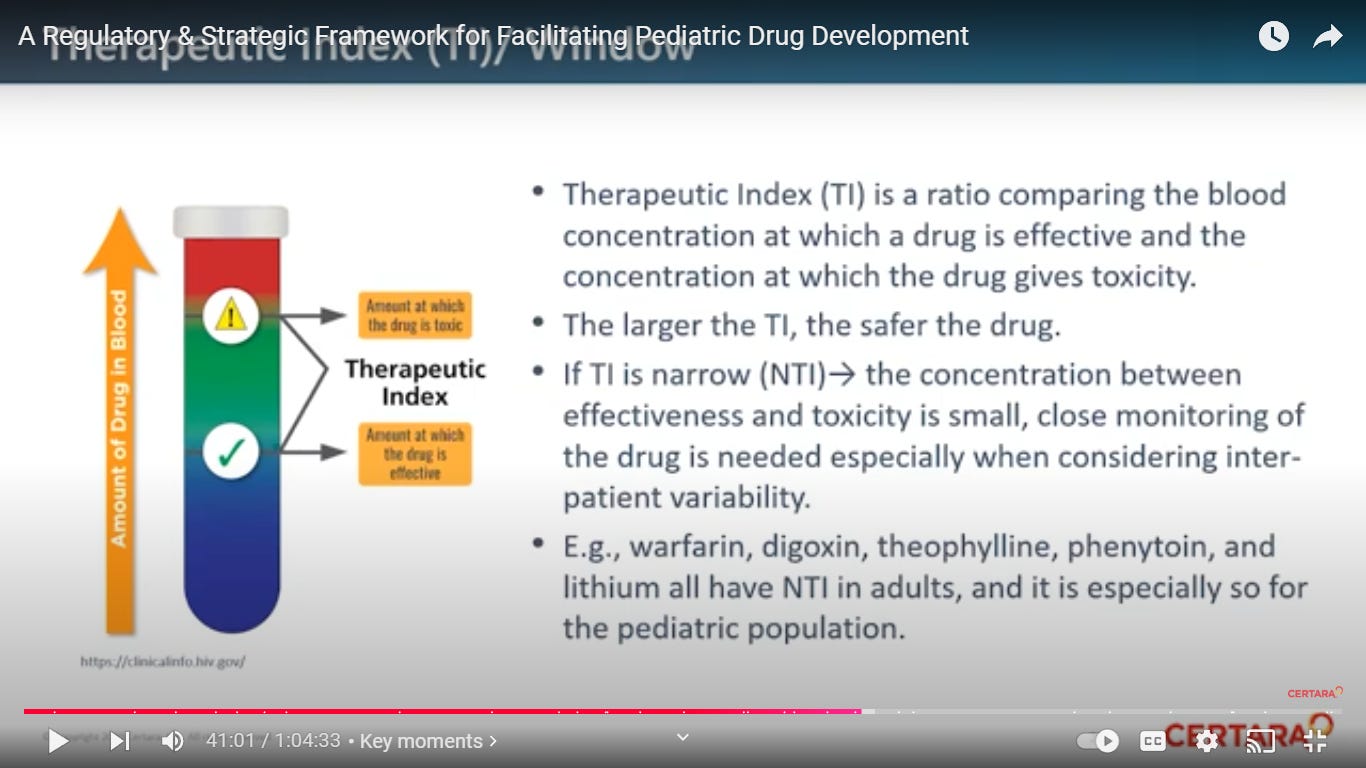
What is a Therapeutic Index? yikes. an NTI means the distance been effectiveness and toxicity is small.
Winning at pediatric drugs
Well I hope this ‘regulatory’ expose creates interest in your trust in the regulators and your children’s health on the ‘approved’ pharmacopia.







What ever happened to the ounce of prevention concept?
And it get worse. Like they haven't lost the publics trust already.