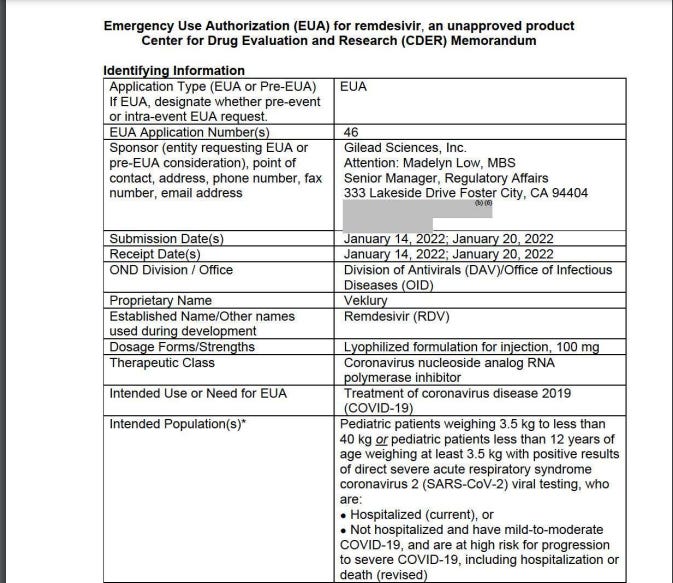
Emergency Use Authorization (EUA) for remdesivir, an unapproved product Center for Drug Evaluation and Research (CDER) Memorandum
HOW DID THEY RATIONALIZE GIVING THIS DRUG TO INFANTS
MODELS AND SIMULATIONS (LIKE CLIMATE CHANGE. THIS IS AL GORE IN PHARMA)
PREDICTED TO BE SIMILAR TO CERTAIN SPECIFIC ADULT STUDIES;
Expectations that DATA ON ADULTS will be the same, and ‘extrapolations’
Phew! that should work for those babies.
“Rationale for Dose in Pediatrics:
The recommended dose for RDV for use in non-hospitalized pediatric patients with mild-to-moderate COVID-19 was based on the following:
• Based on pharmacokinetic modeling and simulation, exposures in pediatric patients after administration of the authorized pediatric dose(s) are expected to be comparable to the exposures observed in adults after administration of the authorized adult dose(s).
• The recommended dosing in hospitalized and non-hospitalized adult and pediatric patients for the approved product is the same except for the duration of therapy (5-10 days for hospitalized and 3 days for nonhospitalized). Therefore, the same principle was used for the pediatric patients 3.5 kg to less than 40 kg or pediatric patients less than 12 years of age weighing at least 3.5 kg. The same dosing under the EUA for hospitalized pediatric patients was used for the revised EUA for non hospitalized pediatric patients except for the duration (5-10 days for hospitalized and 3 days for non-hospitalized).
• Robust clinical data are available in hospitalized and non-hospitalized adults. Because exposures in pediatric patients are expected to be comparable to the exposures observed in adults, the EUA revision for non-hospitalized pediatric patients with mild-to-moderate COVID-19 is supported through extrapolation of efficacy from adequate and well-controlled studies in adults. “
and now we get to the REASONABLE TO BELIEVE APPROVAL SECTION AND RISK BENEFIT SECTION
“VI. Risk-Benefit Assessment and Recommendations for Emergency Use Based on the totality of scientific evidence available, it is reasonable to believe that Veklury may be effective for the treatment of COVID-19 in pediatric patients weighing 3.5 kg to less than 40 kg or pediatric patients less than 12 years of age weighing at least 3.5 kg, with positive results of direct SARS-CoV-2 viral testing, who are not hospitalized and have mild-to-moderate COVID-19, and are at high risk for progression to severe COVID-19, including hospitalization or death, and that, the known and potential benefits of Veklury, when administered for the treatment of COVID-19 as described above, outweigh the known and potential risks of the product.”
REASONABLE TO BELIEVE IT MAY BE EFFECTIVE. AND MAY BE SAFE FOR INFANTS.
BASED ON CLIMATE CHANGE LIKE MODELS. * my designation. not theirs. Interpret differently. Go ahead.
“Although the available data have limitations, it is plausible that the impact of a direct-acting antiviral drug (such as remdesivir) for the treatment of COVID-19 may be greater if administered earlier in the disease course when viral replication, as opposed to immunopathological processes, may be playing a greater role.
FDA has determined that expanding this authorized use is appropriate to protect public health or safety under section 564(g)(2) of the Federal Food, Drug and Cosmetic Act. The Agency believes that expanding the EUA will ensure that important information about the recommended use (e.g., dosing recommendations) for pediatric patients not covered under the USPI will continue to be available to health care providers. As noted above, FDA will revise the EUA concurrent with the approval of NDA 214787/S-10.”
warnings though
Warnings
There are limited clinical data available for VEKLURY in pediatric patients
weighing 3.5 kg to less than 40 kg or pediatric patients less than 12 years of age
weighing at least 3.5 kg. Serious and unexpected adverse events may occur that
have not been previously reported with VEKLURY use.
Hypersensitivity Including Infusion-Related and Anaphylactic Reactions
Hypersensitivity reactions, including infusion-related and anaphylactic reactions,
have been observed during and following administration of VEKLURY; most
occurred within one hour. Signs and symptoms may include hypotension,
hypertension, tachycardia, bradycardia, hypoxia, fever, dyspnea, wheezing,
angioedema, rash, nausea, diaphoresis, and shivering. Slower infusion rates,
with a maximum infusion time of up to 120 minutes, can be considered to
potentially prevent these signs and symptoms. Monitor patients during infusion
and observe patients for at least one hour after infusion is complete for signs and
symptoms of hypersensitivity as clinically appropriate. If signs and symptoms of a
clinically significant hypersensitivity reaction occur, immediately discontinue
administration of VEKLURY and initiate appropriate treatment. The use of
VEKLURY is contraindicated in patients with known hypersensitivity to VEKLURY
or any components of the product [see Full EUA Prescribing Information,
Contraindications (4), Warnings and Precautions (5.1)].
Increased Risk of Transaminase Elevations
Transaminase elevations have been observed in healthy volunteers who
received 200 mg of VEKLURY followed by 100 mg doses up to 10 days; the
transaminase elevations were mild (Grade 1) to moderate (Grade 2) in severity
and resolved upon discontinuation of VEKLURY. Transaminase elevations have
6
Reference ID: 4924711
also been reported in patients with COVID-19 who received VEKLURY. Because
transaminase elevations have been reported as a clinical feature of COVID-19,
and the incidence was similar in patients receiving placebo versus VEKLURY in
clinical trials of VEKLURY, discerning the contribution of VEKLURY to
transaminase elevations in patients with COVID-19 can be challenging [see Full
EUA Prescribing Information, Warnings and Precautions (5.2)].
Perform hepatic laboratory testing in all patients before starting VEKLURY and
during treatment as clinically appropriate.
• Consider discontinuing VEKLURY if ALT levels increase to greater than
10 times the upper limit of normal.
• Discontinue VEKLURY if ALT elevation is accompanied by signs or
symptoms of liver inflammation.
Risk of Reduced Antiviral Activity When Coadministered with Chloroquine
Phosphate or Hydroxychloroquine Sulfate
Coadministration of VEKLURY and chloroquine phosphate or
hydroxychloroquine sulfate is not recommended based on data from cell
culture experiments demonstrating a potential antagonistic effect of chloroquine
on the intracellular metabolic activation and antiviral activity of VEKLURY [see
Full EUA Prescribing Information, Warnings and Precautions (5.3), Drug
Interactions (10), Microbiology/Resistance Information (15)].
Serious Side Effects
Serious adverse reactions have been associated with VEKLURY [see Full EUA
Prescribing Information, Overall Safety Summary (6.1)].
Additional serious adverse reactions associated with the drug may become
apparent with more widespread use.”
WHO SIGNED THIS FOR BABIES?
This is a representation of an electronic record that was signed electronically. Following this are manifestations of any and all electronic signatures for this electronic record. /s/
SAEBYEOL JANG 01/21/2022 03:57:30 PM
KIMBERLY A STRUBLE 01/21/2022 04:00:21 PM
YODIT BELEW 01/21/2022 04:04:09 PM
JOHN J FARLEY 01/21/2022 04:07:04 PM
for you: here is the whole Memorandum downloaded
Please share and review. How do you feel about EAU approved products for infants?
Shitty?
Ya. Me too.



The Remdesivir issue is like all others …. we seem to be trying to ‘live’ in our old (far more pleasant & comfortable) past beliefs. However, the last 5 painful years is now clearly reveals we’ve been living in a FALSE sense of security. We need to comprehend and accept that all the regulatory bodies are corrupt (as above 👆🏻) not only do they NOT protect humans, they have been weaponized through lawfare to hurt/harm/kill (or aid and abet in killing), with directions from our de facto NOT de juer governments (that aren’t governments at all BUT they are corporations (look it up on EDGAR) that are United Nations City states (yes, run by the UN), hence why “Operation LOCKSTEP” was so effective.
GOD bless G. Edward Griffin (The United Nations, 1964; Creature from Jekyl Island, 1994 with his Red Pill University Expos - that every media/internet system censors down to a near zero find ability) for screaming our current realities in his 1st book 60 years ago on the U.N., for James Roguski currently for keeping the IHR on everyone’s radar.
We must STOP seeking these ‘governmentesque’ corporations to save us - and WORSE, unfortunately, is that also means the ‘law societies’ and their regulatory bodies will NOT be the final bastion to save us.
We MUST develop a new system completely, the current one is NOT salvageable ……. BURN the regulators to the ground - follow Dr William Makis on X to see how the Alberta College of Physicians treats him and now Danielle Smith’s court system wants him imprisoned …. this type of thuggery goes WAAAAAY back - look how they went after Dr Judy Mikovits and what ‘they’ (media & internet) said and still say about her now.
WE, the ‘people’ (but not the definition of ‘people’ found in black’s Law Dictionary) The masses, need to be better read and informed and ready to PIVOT
Thanks go4 ALL of your posts !!! Unfortunately, the under belly of law/lawyers indoctrination at law schools (vs being taught lawful not legal), needs to be exposed just like medicine just got eviscerated during the plandemic ….
🤞🏻. 🫶🏻
I don't see what the emergency is? Doesn't an EUA get authorized only when there is no other treatment available? I understand the chances of a child getting covid are miniscule. Sounds like another pharma grift to me.