SO FIRST THEY RUN a study on vaccine confidence in the EU in 2018.
Then the EU publishes and sets out the Immunisation Information System with clear legal and regulatory guidance in 2018.
Then they host the Global Vaccination Summit in the EU in Brussels September 2019.
Then they publish TEN ACTIONS TOWARDS VACCINATION FOR ALL in November 2019.
In December 2019 China has it’s first reported Covid case.
This is not an organic ‘Pandemic’. It is an orchestrated careful administration of vaccines for all. My first article is set out below if you missed it. This article deals with the Technical Report on the Immunization Information System, which functioned very much like we experienced. The downloads and links are for your more detailed perusal, because there is intense country specific information beyond the EU.
Then they run the technical report on setting up the Vaccine Information System.
The EU in 2018 created the following Technical Report regarding Implementing an IMMUNISATION INFORMATION SYSTEM. nothing. nothing. left to chance.
European Centre for Disease Prevention and Control. Designing and implementing an immunisation information system. Stockholm: ECDC; 2018. Stockholm, November 2018 ISBN 978-92-9498-274-2 doi: 10.2900/349426 Catalogue number TQ-01-18-982-EN-N © European Centre for Disease Prevention and Control, 2018
ECDC TECHNICAL REPORT Designing and implementing an immunisation information system A handbook for those involved in the design, implementation or management of immunisation information systems
Designing Implementing Immunisation Information System 0
2.18MB ∙ PDF file
Who is working on this SYSTEM TO COLLECT US ALL. one ring. Look for your Country’s contributors.
This report was commissioned by the European Centre for Disease Prevention and Control (ECDC) and coordinated by
Tarik Derrough.
Kate Olsson (ECDC) provided extensive contribution to the technical guidance and facilitated liaison with contributing experts. We would also like to acknowledge the contribution of
Karam Adel Ali, Laszlo Balkanyi,
Lucia Pastore-Celentano,
Niklas Danielsson,
Andrea Iber,
Svetla Tsolova and Piotr Kramarz for supporting this work and providing expert input to its content.
Acknowledgements
ECDC would like to especially acknowledge the invaluable input received from
Sigrun Kongsrud (Norwegian Institute of Public Health and Environment, Oslo) and Rebecca Coyle (American Immunization Registry Association, Washington, DC) in guiding the development of this document.
ECDC would like to acknowledge the support and guidance provided by members of the ECDC technical expert group on immunisation information systems and other experts who contributed to this technical document, discussed its outline, provided content during face-to-face meetings at ECDC, or provided examples of case studies:
Ana María Alguacil-Ramos (Conselleria de Sanidad Universal y Salud Pública, Valencia Regional Health Authority, Spain),
Silvia Bino (National Institute of Public Health Albania),
Christoph Bornhöft (Germany),
Claire Cameron (Health Protection Scotland, NHS National Services Scotland, Scotland, UK),
Niyazi Cakmak (World Health Organisation Regional Office for Europe, Denmark),
Marcela Contreras (Pan American Health Organization, USA),
Fortunato ‘Paolo’ D'ancona (National Public Health Institute, Italy),
Siddhartha Datta (World Health Organisation Regional Office for Europe, Denmark),
Jan Grevendonk (World Health Organization, Switzerland),
Hans Jürgen Dornbusch (Austria),
Michael Edelstein (Public Health England, UK),
Adamos Hadjipanayis (Cyprus),
Maria Hagerup-Jenssen (Norwegian Institute of Public Health, Oslo),
Harald Heijbel (Swedish Institute for Infectious Disease Control, Stockholm),
Lucy Jessop (Public Health Agency, Northern Ireland),
Ziad El-Khatib (Karolinska Institutet, Sweden),
Tyra Grove Krause (Statens Serum Institut, Denmark),
Jean-Louis Koeck (Hopital Robert Picqué, France),
Aurora Limia Sanchez (Ministry of Health, Spain),
Elena Moya (Spain),
Apophia Namageyo-Funa (Centers for Disease Control and Prevention, USA),
Christian Perronne (Raymond Poincaré University Hospital, France),
Marvin Philippi (National Institute for Public Health and the Environment, Netherlands),
Borna Pleše(Croatian National Institute of Public Health, Croatia),
Iria Preza (National Institute of Public Health Albania),
Thorsten Rieck (Robert Koch Institute, Germany),
François Simondon (Research Institute for Development, France),
Niamh Sneyd (National Immunisation Office, Ireland), J
onas Sundman (National Institute for Health and Welfare, Finland),
Jean-Pierre Thierry (France), Karen Tiley (Public Health England, UK),
Joanne White (Public Health England, UK),
Laurie Werner (PATH, USA),
Joel Willis (Department of Health, Australia),
Kumanan Wilson (Ottawa Hospital, Canada),
Mary Woinarowicz (North Dakota Department of Health, USA),
Irmgard Zonnenberg (National Institute for Public Health and the Environment, Netherlands).
ECDC would also like to acknowledge Germano Ferreira and Sally Jackson (P95) who were contracted out for supporting the production of this technical guidance.”
WHAT DID THE BOOKLET CONTENT CONTAIN?
Contents
Abbreviations..............................................................................................................................v
Glossary.......................................................................................................................................vi
Country codes..............................................................................................................................vi
Purpose ........................................................................................................................................................1
Overview......................................................................................................................................2
Section 1. The context..............................................................................4
1.1 Overview...........................................................................................................4
1.2 Defining immunisation information systems ...............................................................4
Terminology..............................................................................................................4
Scope ..................................................................................4
Distinction between Electronic Immunisation Registries (EIR) and IIS........................5
Functionalities ............................................................................................5
1.3 Purpose of immunisation information systems..................................................5
Provide information to make better operational decisions ................................................6
Provide information to make better informed strategic decisions ...............................6
1.4 Global policy and practice .........................................................................7
1.5. Information Technology, eHealth, and IIS ................................................................8
EHealth in the EU .............................................................................................8
Integration of IIS in health information exchanges ............................................9
Patient engagement...............................................................................10
Section 2. IIS functions and value ..............................................................................12
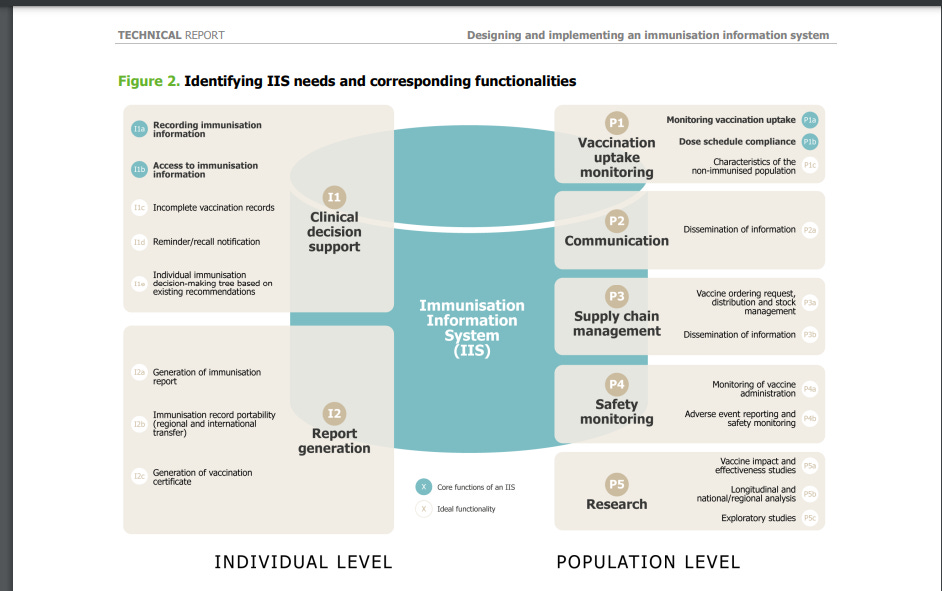
2.1. The need for an IIS and its functionalities ................................................................12
2.2. Value of IIS for stakeholders ..................................................................13
2.2.1. The individual ...................................................................................14
2.2.2. Healthcare professionals and vaccine providers ..........................................15
2.2.3. Vaccine programme managers and policymakers..................................................17
2.2.4. Health researchers ....................................................................................21
Section 3. An enabling environment for IIS .................................................23
3.1. The regulatory environment: Legal and data protection ............................................23
3.2. Implementing the General Data Protection Regulation (GDPR) ..............23
Legal principles for processing of personal health data..................................23
Implementing the GDPR........................................................................................24
3.3. Principles of the use of IIS data for the benefit of individuals, public health, and scientific research.....25
IIS and benefits to individuals and populations.......................................26
Country-specific examples of practices..............................................................26
Informed consent upon record creation .................................................................27
Informed consent upon application download.................................................................27
Section 4. System design and development..................................................................28
4.1. High-level considerations ........................................................................28
Fundamental differences between different European contexts exist in terms of legal regulations, IIS management, and user incentives. ................................................29
4.1.1. A ‘top-down’ centralized approach ...................................................29
4.1.2. A ‘bottom-up’ approach................................................................................31
4.2. System-level considerations ......................................................................33
4.2.1. Recording immunisation event data .....................................................33
4.2.2. Data elements....................................................................................36
4.2.3. Establishing the denominator ..........................................................................43
4.2.4. Ensuring data quality ....................................................................44
4.2.5. Storing data...........................................................................................46
4.2.6. User access ........................................................................................47
4.2.7. Linkage to other information systems and interoperability ...............................48
Designing and implementing an immunisation information system TECHNICAL REPORT
iv
Section 5. Project planning for sustainability..................................................................52
5.1 Project management and leadership ......................................................................52
Interdisciplinary team ...........................................................................52
Mapping the context ................................................................................52
Making a high level but comprehensive conceptual model of the system..................53
An iterative approach..............................................................................................55
Approaches for system roll-out ........................................................................55
Training users of the system............................................................................55
Performance and sustainability..................................................................56
5.2 Challenges to anticipate.............................................................................56
Learnings from the ECDC survey ...................................................................56
Different system development options are used in Europe...............................................58
Balancing costs against benefits and acquiring sustainable funding........................59
Concluding remarks ..................................................................................61
Annex. National IIS, immunisation registers and systems in pilot testing............................................62
Abbreviations
AEFI Adverse events following immunisation
AIRA American Immunization Registry Association
API Application program interface
CRVS Civil registration and vital statistics
DDV Danish vaccination register
ECDC European Centre for Disease Prevention and Control
EAP European Academy of Paediatrics
EHR Electronic health record
EMA European Medicines Agency
EPR Electronic patient record
EU/EEA European Union/European Economic Area
EVAP European Vaccine Action Plan
GDPR General Data Protection Regulation
GP General practitioner
GVAP Global vaccine action plan
HIE Health information exchange
IIS Immunisation information system
IRD Institut de recherche pour le développement
MMR Measles, mumps and rubella vaccine
MoH Ministry of health
MIC Mass immunisation campaign
NHS National Health Service England
NIPH National institute of public health
NITAGs National immunisation technical advisory groups
NUTS Nomenclature of territorial units for statistics
PAHO/WHO Pan American Health Organization/World Health Organization
RHA Regulatory health authorities
RIPH Regional institute of public health
SIP Session initiation protocol code
RN Registered nurse
SIA Supplementary immunisation activities
SIV The vaccine information system of the Valencia region in Spain
SKOS Simple knowledge organisation system
SNOMED CT Systematized nomenclature of medicine – clinical terms
SSI Statens Serum Institute
SVEVAC Swedish immunisation information systems
SYSVAK Norwegian immunisation registry
US CDC United States Centers for Disease Control and Prevention
VIS Vaccine information systems
WHO World Health Organization
Glossary
eHealth
European Commission definition: the use of ICTs in health products, services and processes combined with organisational change in healthcare systems and new skills, in order to improve health of citizens, efficiency and productivity in healthcare delivery, and the economic and social value of health [1]
mHealth
European Commission definition: mobile health is a sub-segment of eHealth and covers medical and public health practice supported by mobile devices. It especially includes the use of mobile communication devices for health and well-being services and information purposes as well as mobile health applications [2]”
YOU JUST remember that everything assembled down to your proof of vaccine on your phone was PLOTTED SEAMLESSLY.
‘In particular, this technical guidance aims to:
• define immunisation information systems,
• provide information on immunisation information systems and their added-value to immunisation programmes,
• share best practices to advocate for immunisation information systems towards main stakeholders,
• describe the functionalities and attributes that immunisation information systems can offer, and
• give step-by-step guidance on the major steps to be considered for the design, implementation, or further development of an immunisation information system.
The process of setting up an IIS is broken down into a series of steps that cover the entire project cycle. For each step this document will:
• highlight key considerations,
• give examples of lessons learned in a variety of contexts, and
• provide references to a suite of more detailed resources on IIS.”
note this next bit, the travel abroad restrictions were preplanned. so no pandemic yet in sight. but the electronic records keeping you locked in were being planned.
the employment restrictions were already being planned. whether you attend college or not was being planned.
“Distinction between Electronic Immunisation Registries (EIR) and IIS
In the literature, EIR and IIS are often used interchangeably. In order to distinguish them from each other and bring clarity, it is relevant to refer to other commonly used terms:
Immunisation record refers to a written record of vaccination history. An appropriate definition of an immunisation record is provided by the CDC [9]: ‘Vaccination records (sometimes called immunisation records) provide a history of all the vaccines you or your child received.
This record may be required for certain jobs, travel abroad, or school registration’. At the individual level, this corresponds to a hard-copy vaccination card; at the community level, it refers to paper-based nominal registries or records. Immunisation records have always formed the basis of any immunisation programme as they allow the recording of the vaccination event.
Electronic immunisation record refers to a digitalised written record of immunisation history.
Having immunisations recorded in electronic form brings a whole set of new opportunities for improving individual and collective vaccination.
Electronic immunisation records are compiled in a database. This collation of records is referred to as an electronic immunisation registry.
The terms ‘records’ and ‘databases’ should not be confused with the diverse functions that can be associated with them. In addition to vaccination history, some of these features will also allow for the registration of other information on individuals, for example age, sex, profession, or risk factors, while other features allow for other details to be included such as a vaccine inventory function to facilitate vaccine ordering.
Electronic immunisation registries can then be part of an immunisation information system that would refer to the collective dimension of the information system and that offers additional functionalities.
Functionalities
The functionalities of IIS are moving beyond simply recording immunisation and towards the inclusion of advanced features within the systems (Section 4) including:
• personalised information on vaccination,
• a communication platform that allows for targeted communication towards healthcare professionals and the public,
• decision support systems for vaccine providers (e.g. automated protocols for vaccination catch-up),
• recording of reasons for refusal of vaccination, and
• adverse event recording.”
So if you need A BOOSTER the system will tell you. If you are REFUSING. THE SYSTEM WILL KNOW AND KNOW WHY. Oh adverse event recording. Oh. I wonder what happened to that intent.
1.3 Purpose of immunisation information systems
“The purpose of an IIS can best be demonstrated through the direct benefits it provides to stakeholders and the wealth of opportunities offered (Section 2).
Immunisation programmes are complex public health interventions that produce major health benefits. They are vulnerable to changes in public confidence and opinion. Recently, immunisation programmes have been subject to controversy that has hampered uptake for some vaccines.
Mitigating this controversy requires real-life demonstration of the efficacy and excellent safety profile of vaccinations within short timeframes.
IIS are an essential tool for the timely documentation of the health benefits of immunisation. Progress in technology has enabled the development of comprehensive nationwide IIS in contexts where legislation to preserve data security and privacy is in place (Section 3).
There is great interest from the public health community and health officials to develop and implement IIS (Section 2). Implementation is nevertheless subject to challenges, as is illustrated in Section 4.
Despite existing hurdles, in 2016, the results of a survey conducted by the European Centre for Disease Prevention and Control (ECDC) showed a positive trend in the implementation of vaccination registries within European countries [10]. Of 27 responding countries of the European Union/European Economic Area (EU/EEA), 21 answered that they have an IIS in operation or being currently piloted, either at the national or subnational levels. Furthermore, of the six remaining countries, four mentioned that they have concrete plans to implement one in the near future [10]”
So the system is necessary to show SAFE AND EFFECTIVE. oh. This next creepy part, says the reason TO DO ALL OF THIS is to get instant access to the Individuals immunization record. SOCIAL CREDIT.
‘The general purposes of an all-encompassing IIS can be detailed as follows:
Provide information to make better operational decisions To support the delivery of the immunisation programme at the point of administration Vaccination schedules are regularly being revised and reviewed in response to newly available vaccines, catch-up campaigns, and the introduction of temporary schedules due to changes in the epidemiological situation, shortages or specific campaigns.
There are a number of stakeholders involved in the delivery of the immunisation programmes in some countries, including general practitioners (GPs), paediatricians, nurses, gynaecologists and school nurses. There has been a corresponding increase in need for specialist advice on vaccine indications and contra-indications, and this can be offered by IIS through clinical decision support systems and through the medical information that can be provided. IIS can provide access to consolidated immunisation data at the time and place where a decision on vaccination is to be made.
IIS can provide other functionalities that support the delivery of immunisation programmes such as vaccine inventory, vaccine supply or reimbursement management functions.
To enable immediate access to individual immunisation history IIS enable the consolidation of potentially fragmented immunisation records of all people immunised by multiple healthcare providers.
Among other functionalities, IIS enable access to complete records of all vaccinations, which makes it easier for the healthcare provider to offer a tailored immunisation service, ensuring that individuals receive recommended vaccines depending on characteristics including their age, occupation and sex.
IIS also give reassurance that the correct vaccine is given at the right time and avoids unnecessary errors such as double administration.”
Double administration. I am laughing. This next part is the RIGHT TO HEALTH. Goodness they love the Orwellian language. Right to health doesn’t include informed consent, or even the right to refuse the jab, so no bodily autonomy in their world. No school, work, border crossing, bus mounting, plane hopping for you. sounds also very Net zero.
OH AND THOSE EQUITY AFFICIANDOS. Equity? It means we are coming for you too.
Also this system will shut down rumors. unfounded ones. of adverse events.
“Equitable access to immunisation is considered a core component of the right to health.
The Global Vaccine Action Plan (GVAP) aims for coverage of target populations to be at least 90% national vaccination coverage with at least 80% vaccination coverage in every administrative unit for all vaccines in the national immunisation programme [11]. Coverage gaps persist between countries and within countries [11,13]. High-quality immunisation information can serve to reduce inequity through the identification of underserved populations. This facilitates improved understanding of the determinants of inequity and access, enabling better targeting of approaches to increase coverage [13]. In addition, the European Regional Vaccine Action Plan, in order to ‘strengthen monitoring and surveillance systems’, proposes to ‘develop and promote the use of new information technologies for collection, transmission and analysis of immunisation data within immunisation information systems that are well integrated with communicable disease and health information systems’ [13].
Vaccine hesitancy, which is most commonly referred to as the delay in acceptance or refusal of vaccines despite availability of vaccine services [15], presents a challenge to attaining high levels of coverage in Europe.
Transparent post-marketing studies, and studies on the impact of immunisation programmes that are independent from commercial interests, are important for increasing public trust in immunisation [12]. IIS can help mitigate potential rumours and unfounded concerns through the provision of evidence, including on adverse events following immunisation [13]. Another example in the UK (Scotland) has been the added use of ‘Onomap’ a computer package which facilitates name recognition to attribute ethnicity to investigate vaccine coverage in different communities, for example within the Polish community in Scotland. In an ECDC survey, ten countries out of 16 (63%) had IIS that could be used to record reasons for refusal or hesitancy to vaccination [10].”
HERE IS THEIR SYSTEMATIC MARCH
Box 3. Key health policies in the EU related to IIS
2011: The Global Vaccine Action Plan (GVAP) 2011–2020 [11]:
Framework approved by the World Health Assembly to achieve the Decade of Vaccines vision by delivering universal access to immunisation.
GVAP builds on the success of the first 10-year strategic framework to realise the potential of immunisation – the Global Immunisation Vision and Strategy 2006–2015.
It proposes six strategic objectives and the actions that will support their achievement.
2011: Council conclusions on childhood immunisation: successes and challenges of European childhood immunisation and the way forward [17].
2014: The European Vaccine Action Plan (EVAP)
2015–2020 [13]: Regional interpretation of GVAP which was developed to address the specific needs and challenges of the WHO European Region.
2014: Council of the European Union conclusions on vaccinations as an effective tool in public health: Conclusions developed at the Employment, Social Policy, Health and Consumer Affairs Council [12].
2017: The Consumers, Health, Agriculture and Food Executive Agency of the European Commission launched the Joint Action on Vaccination [16] for EU Member States and included IIS as one work area.
2018: Proposed council recommendation on strengthened cooperation against vaccine-preventable diseases [18].”
“The Commission stated that they wanted to
• unlock EU added value for individuals, patients and researchers,
• work with interested Member States to ensure secure cross-border transfer of health records electronically,
• use e-prescriptions to dispense individuals’ medication when people are travelling abroad,
• promote high-performance computing capacity to unlock the potential of big data for health through advanced data analytics such as in areas of development of medicines and early detection of emerging infectious diseases.
In its mid-term review of the DSMS, the Commission’s Communication on the Transformation of Digital Health and Care of April 2018 identified three pillars around which activities will be based [19,23]:
• Secure access to, and sharing of, electronic health data
• Connecting health data to advance research, prevention and personalised medicine
• Using digital tools to foster citizen empowerment and person-centred care.
IIS may not only benefit from digital health development but their wider deployment may also contribute to the Commission’s three pillars for digital health and care in the following ways:
• IIS have a relatively long history of development and standardisation, starting initially with registries, which makes IIS more mature than other population-based health information systems, and one of the leaders in
population-based data and healthcare delivery.
• Data collected within IIS have been used in research to inform public health decisions.
• IIS would allow individuals to access their immunisation history and thereby take ownership and manage
their vaccination history. Clinical decision support systems allow immunisations to be tailored and adjusted
based on individual determinants including age, occupation, medical history and prior immunisation history
Designing and implementing an immunisation information system. On the 12th September 2019, at the joint EU-WHO “Global Vaccination Summit”, they announced a “10 Actions Towards Vaccination for All” plan, to
“Examine the feasibility of developing a common vaccinationcard/passport for EU citizens“
“Develop EU guidance for establishing comprehensive electronic immunization information systems for effective monitoring of immunization programmes.”
“overcome the legal and technical barriers impeding the interoperability of national immunisation information systems”
Health information exchange (HIE) refers to the movement of health information among different health information systems. In Europe, IIS have been established that centralise information, facilitating the development of common software and data consolidation, and enabling the linkage of data at both the patient and population level. Internationally, the need for a standardised patient identifier across multiple information systems is increasingly recognised, and HIE is a component of most eHealth system plans at the regional and country levels.
An interoperability framework is being developed internationally [26], although differences persist at the national level. Increasingly, consideration of, and compliance with, the framework is expected of technology providers.
Compared with centrally managed networks, standalone platforms such as mobile applications can be developed rapidly. Many independent developers bring IIS solutions to the market, providing them directly to the consumer.
While these independent products address an immediate need, their added value will often be realised through connection to the wider health system: integration of a mobile application into servers or platforms at the regional/national level can slow the diffusion of the app in the short term, but in the longer term, integration yields benefits through the workflow of data. The extensive use of such a tool can increase the chances for its longevity.
Technical tools for the integration of the technology within a multi-source information platform are available to facilitate HIE across health and social care settings, but developing a centralised IIS and integrating multiple data streams can be complex due to fragmentation, which is present in existing systems in many countries. For example, in some countries (e.g. France), vaccination involves many different actors and health structures (e.g. family doctors, pharmacists, nurses or midwives, medical offices, hospitals, occupational health services, health centres or retirement homes, school settings, national and regional health policymakers). In addition, some vaccines will be administered to the patient outside of the regular health system channels (e.g. in supermarkets). It is also important to be able to transfer/upload immunisation data to other databases, for example HPV vaccination records to cervical screening databases. It is very complex to harmonise all of these data and information. In other countries such as Sweden, registries are not consolidated at the national level and no multi-source information system is capable of managing all aspects of immunisation.”
It is no wonder the Gates team and organizations are involved in this project.
THE US EXAMPLE
“Box 5. The Blue Button Project
A US-based government initiative whereby individuals can, in just one click, get their health information online; downloading their health records in a variety of formats (e.g. text or .pdf) on supported computers and smartphones. Multiple organisations feature ‘Blue Buttons’ on their websites to enable individuals to access their personal medical information and claims. This gives individuals unprecedented control and management over their own wellbeing [30]. “
what do you think of the Blue Button project if you are in the US?
“In addition to individual benefits, patient generated data can complement healthcare systems and improve public health overall [28,32]: • Two-way communications through the IIS platform can potentially minimise vaccine hesitancy, both through the rapid reporting of health effects and through targeted information dissemination. • In addition to monitoring information on vaccines delivered and documenting risks or adverse events associated with vaccination, interactive immunisation records can also provide double validation of data (provider and patient), which is also likely to improve data quality and completeness.”
Who is the creepiest on the population level vote in the comments.
the health researcher
the vaccination programme managers;
the policy makers; or
the Health Authority.
Section 3.
An enabling environment for IIS In this section we:
• describe the legal environment for IIS;
• consider the implementation of the General Data Protection Regulation (GDPR) both across and within countries, particularly focusing on consent; and
• outline the principles of the use of IIS data for the benefit of individuals, public health, and scientific research.
It is important to have regulations governing IIS in place before starting to implement an IIS. We recommend careful consideration of the legal context for IIS at an early stage, ensuring that it commences before system development so that data protection principles can be built into system design.
3.1. The regulatory environment:
legal and data protection In 2012, the European Commission initiated a comprehensive reform of the EU’s 1995 data protection directive 95/46/EC [55] due to the following facts:
• Rapid technological and business developments have brought new challenges for the protection of personal data.
• The 27 EU Member States had implemented the 1995 rules differently, resulting in divergences in enforcement [56].
Within the common EU rules, the general data protection principles remain unchanged, but are translated into the context of modern technological developments. They aim to:
• strengthen individual fundamental rights in the digital age;
• facilitate business by simplifying rules for companies in the digital single market; and
• reduce fragmentation and administrative burdens [57].
The reformed EU rules provide more clarity on definitions and facilitation of the use of health data for scientific research, public health and social security systems. This has a direct impact for data that will be contained within an IIS.
The EU-wide data protection instrument, the General Data Protection Regulation - Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) [58] entered into force on 24 May 2016 and became directly applicable on 25 May 2018 [59]. Some key features of the General Data Protection Regulation (GDPR) are given in Box 21.
The official text of the Regulation was published in the Official Journal of the European Union on 4 May 2016. Box 21. Key features of regulation (EU) 2016/679
• Single and comprehensive set of rules in all Member States
• Reinforced rights of individuals, including the ‘right to be forgotten’ • Same rules for entities based within and outside the EU
• The establishment of a one-stop-shop mechanism for businesses and individuals • Promotion of ‘privacy by design’
• New safeguards: privacy impact assessments, mandatory DPOs.
3.2. Implementing the General Data Protection Regulation (GDPR)
Legal principles for the processing of personal health data Under the GDPR, processing of personal health data is generally prohibited, unless it falls into one of the expressly foreseen scenarios in Articles 6 and 9 of the GDPR (i.e. there is a ‘legal basis’) [58] .
‘Personal data’ refers to any information relating to an identified or identifiable natural person; personal data can be either: Designing and implementing an immunisation information system TECHNICAL REPORT 24
• pseudonymised: data that can no longer be attributed to an individual without the use of additional information; or
• anonymised: data that can no longer be linked to an individual. Accordingly, processing of personal health data is legal if it is for individual level benefit, through informed consent; or in specific situations where consent is not required.
For the purpose of IIS, no consent may be required in situations where processing is necessary, referred to in Chapter 11: Processing of special categories of personal data, Article 9, paragraph 2 [58]:
• to protect the vital interests of the data subject or of another natural person where the data subject is physically or legally incapable of giving consent;
• for the purposes of preventive or occupational medicine, for the assessment of the working capacity of the employee, medical diagnosis, the provision of health or social care or treatment or the management of health or social care systems on the basis of a law or pursuant to contract with a health professional;
• for reasons of public interest in the area of public health, such as protecting against serious cross-border threats to health or ensuring high standards of quality and safety of healthcare and of medicinal products or medical devices; and
• for archiving purposes in the public interest, scientific or historical research purposes or statistical purposesIn all these scenarios, a number of general principles should be respected.
In particular, the data should be:
• collected for specified, explicit and legitimate purposes; • adequate, relevant and not excessive in relation to this purposes;
• accurate and, where necessary, kept up to date; • kept in a form which permits identification of individuals for no longer than is necessary. Accordingly, the following consent considerations should be taken into account when processing health data:
• Processing with consent: An individual needs to provide active, informed consent for data to be collected. Silence, pre-ticked boxes, and inactivity do not constitute consent. Consent can be broader to cover permission for pre-defined scientific research purposes (Recitals 32 and 33, Article 7 of the GDPR). The individual should also be informed why the data are collected, who has access, how long they are kept and where he/she can turn to in case of complaints.
• Processing without consent: In many cases, it is required that data processing is conducted by a health professional under professional secrecy or social security systems.
In other cases, measures should be taken to limit the amount of personally identifiable information by applying anonymisation or pseudonymisation techniques.
For example: − Individual-level identifiable data may be processed without consent when vital to the data subject. Only the data strictly necessary for this purpose should be processed and preferably this information should be handled by health professional under professional secrecy. − If data are processed because it is necessary for the interests of public health or for scientific research, the data should be (pseudo)anonymised as far as possible
Implementing the GDPR
The general data protection principles of the GDPR are largely the same as in the Directive, but there is improvement in harmonisation of the data definitions, including clarifications of the notions of ‘personal data’, ‘consent’, ‘data concerning health’, ‘genetic data’, and ‘biometric data’.
The GDPR also introduced the new concepts of ‘profiling’ and ‘pseudonymisation’. General principles of the GDPR need to be linked to the specific needs of the IIS. Member States will need to assess the provisions in pre-existing national laws, and sectoral laws (e.g. for health data) to see if existing legislation can be applied.
The GDPR is directly applicable in the national legal system, therefore no implementation measures are needed. However, when references are made to specifications/restrictions by Member State law, the Member State may incorporate elements in their national law, as noted in particular in Article 9(4): ‘Member States may maintain or introduce further conditions, including limitations, with regard to the processing of genetic data, biometric data or data concerning health’. ‘Health Data’ refers to data collected in the context of provision of healthcare services as defined in Directive 2011/24/EU (Recital 35 of the GDPR)
Box 25. Informed consent for CANImmunize In Canada,
CANImmunize [60] is an application (mobile and web) that allows individuals to create accounts within which they can store immunisation records for themselves and their family members. Before a user can create an account, they are presented with a screen which describes in plain language how the application works. This includes sections on what data the app collects, how they are protected, how users can delete their information, and under what conditions the information entered by the user is shared with third parties. The user is also directed to review a comprehensive privacy policy and terms of use, which they must accept before being allowed to access the application. CANImunize can share data with other IIS, but users must always provide an additional layer of consent indicating that they allow CANImmunize to share their immunisation records with the trusted third-party software before data are exchanged.
4.2. System-level considerations
There are a number of different considerations to be taken into account when approaching system design (Table 1).
Table 1. System-level design considerations
System-level design Considerations
Existing system(s) available
It is important to consider how other existing systems can be leveraged to build a comprehensive IIS, as opposed to duplicating efforts and building a new system from scratch.
Spatial scale Systems can be centralised (national) or decentralised, for example regional systems.
Users If there is a diversity of users of the system (e.g. nurses, GPs etc.) and vaccine delivery is complicated (e.g. different settings), then the system will need to be more complex to capture this.
Inputs/Outputs Systems can incorporate one or more input systems. These systems can then communicate to one or more other systems.
Vendors
One or more vendors of one or multiple systems.
Ownership
All private, all public, or a public-private mix.
System interface Choices include, for example, web or mobile applications.
Country-context-specific factors that will affect design decisions include:
• System purpose and objectives
• Population size
• Government
• Legal limitations
• Infrastructure
• Funding
• Availability of data in other systems, for example a unique identifier number
• eHealth strategy
Most importantly, systems should aim to produce data of high quality i.e. data that is complete, reliable, and accurate. Importantly, all systems should be built with sustainability in mind (see Chapter 5).
When designing an IIS, the following elements, which are outlined in detail on the following pages, should be considered carefully:
• Recording immunisation event data
• Data elements
• Establishing the denominator
• Ensuring data quality
• Storing data
• User access
• Linkage to other information systems and interoperability”
Box 35. Standardised terminology for Canada In Canada, standardised terminology for immunisations has been created in an effort to promote the standardisation of immunisation records and IIS. Canada Health Infoway maintains the Canadian SNOMED CT extension through which SNOMED CT [70] codes can be created for concepts specific to the Canadian context. Two subsets of immunisation concepts were created: one to identify administered vaccines where the vaccine product is known, and another to identify historical vaccination records where the vaccine product is unknown. This terminology is used in a number of IIS and other applications in Canada, including CANImmunize, and is now included in the Canadian Immunisation Standard [78]. The relationships component of SNOMED CT is also used to facilitate mapping between the historical and trade name concepts, which is useful for immunisation forecasters.
Box 40. Barcode scanning within CANImmunize in Canada and MesVaccins.net in France
Canada: Early in the development of the CANImmunize app, it was identified that vaccine barcode scanning may serve as a more efficient mode of data entry to free text entry of vaccine names and product information.
Barcode scanning has also been shown to improve the data quality of vaccination records. Canada has since mandated that vaccine manufacturers print a 2D DataMatrix barcode containing global trade item number (GTIN), lot number, and expiry date on the vaccine vial and secondary packaging.
Allowing CANImmunize users to scan these barcodes using an app can enhance vaccine safety. For example, users who received a dose of a recalled lot could be contacted or identified through the CANImmunize app. A study was conducted to evaluate the efficacy of using mobile devices to scan vaccine vial barcodes. It was found that barcode scanning was feasible as a means for data entry during a vaccination visit [80].
While barcode scanning itself was feasible, clinical workflow presented a challenge to implementation. It was also identified in the course of this work that Canada lacked a source-of-truth database containing vaccine product identifiers (GTIN, lot number) mapped to the vaccine concept identifiers (SNOMED CT), which are required to store the immunisation record in an IIS conforming to the Canadian Immunisation Standard [78].
The lack of such a data source would make it difficult to maintain barcoding functionality that could identify all vaccines. More work is needed to realise the potential of barcodes in the context of immunisation apps for the public.”
I have only grabbed a smattering of information over a 77 page report. Please review. If you think the pandemic was an organic experience that we contended with on the fly, you need more IQ points.
This was the implementation of a solution that the pandemic provided. I note that it states ‘government’ as a factor. You need the ‘right government actors’ in place. aka WEF placements.
The next thing the EU did was host a GLOBAL VACCINATION SUMMIT BRUSSELS, 12 SEPTEMBER 2019
And publish TEN ACTIONS TOWARDS VACCINATION FOR ALL
“Everyone should be able to benefit from the power of vaccination. Despite the availability of safe and effective vaccines, lack of access, vaccine shortages, misinformation, complacency towards disease risks, diminishing public confidence in the value of vaccines and disinvestments are harming vaccination rates worldwide. Vaccination is indisputably one of public health’s most effective interventions. We must endeavor to sustain vaccination’s hard-won gains but also aim to do more and to do better, in view of achieving effective and equitable health systems and reduce the harm that is caused as a result of the illness and suffering that is otherwise preventable. This also includes making the necessary R&D investments to address unmet medical needs by developing new vaccines and improving existing ones
Lessons from the day and actions needed towards vaccination for all and elimination of vaccine preventable diseases:
1. Promote global political leadership and commitment to vaccination and build effective collaboration and partnerships -across international, national, regional and local levels with health authorities, health professionals, civil society, communities, scientists, and industry- to protect everyone everywhere through sustained high vaccination coverage rates.
2. Ensure all countries have national immunisation strategies in place and implemented and strengthen its financial sustainability, in line with progress towards Universal Health Coverage, leaving no one behind.
3. Build strong surveillance systems for vaccine-preventable diseases, particularly those under global elimination and eradication targets.
4. Tackle the root-causes of vaccine hesitancy, increasing confidence in vaccination, as well as designing and implementing evidence-based interventions.
5. Harness the power of digital technologies, so as to strengthen the monitoring of the performance of vaccination programmes.
6. Sustain research efforts to continuously generate data on the effectiveness and safety of vaccines and impact of vaccination programmes.
7. Continue efforts and investment, including novel models of funding and incentives, in research, development and innovation for new or improved vaccine and delivery devices.
8. Mitigate the risks of vaccine shortages through improved vaccine availability monitoring, forecasting, purchasing, delivery and stockpiling systems and collaboration with producers and all participants in the distribution chain to make best use of, or increase existing, manufacturing capacity.
9. Empower healthcare professionals at all levels as well as the media, to provide effective, transparent and objective information to the public and fight false and misleading information, including by engaging with social media platforms and technological companies.
10. Align and integrate vaccination in the global health and development agendas, through a renewed Immunisation agenda 2030.
https://health.ec.europa.eu/document/download/389a127e-39e1-4989-8647-0e0777c0dec3_en
The electronic ownership of humans is diabolical. This post should push back on even the most gullible of narrative followers. I can’t see anyone with an ounce of critical reasoning having the ability to read through this and believe that everything took place in an organic fashion.
The EU is the center in some ways isn’t it.
TAKE DOWN THE BEAST.








A rigged system that only criminals benefit.
I'm looking at Propaganda, by Jacques Ellul. On p. 124 there is an interesting footnote.
"The Soviet Union, despite its authoritarian character and the absence of opinion surveys, makes just as much effort to keep informed of public opinion -- through agitators (who inform the government on the people's state of mind) and through letters to the press. The government does not consult opinion in order to obey it, however, but to know at what level it exists and to determine what propaganda action is needed to win it over."